Abstract
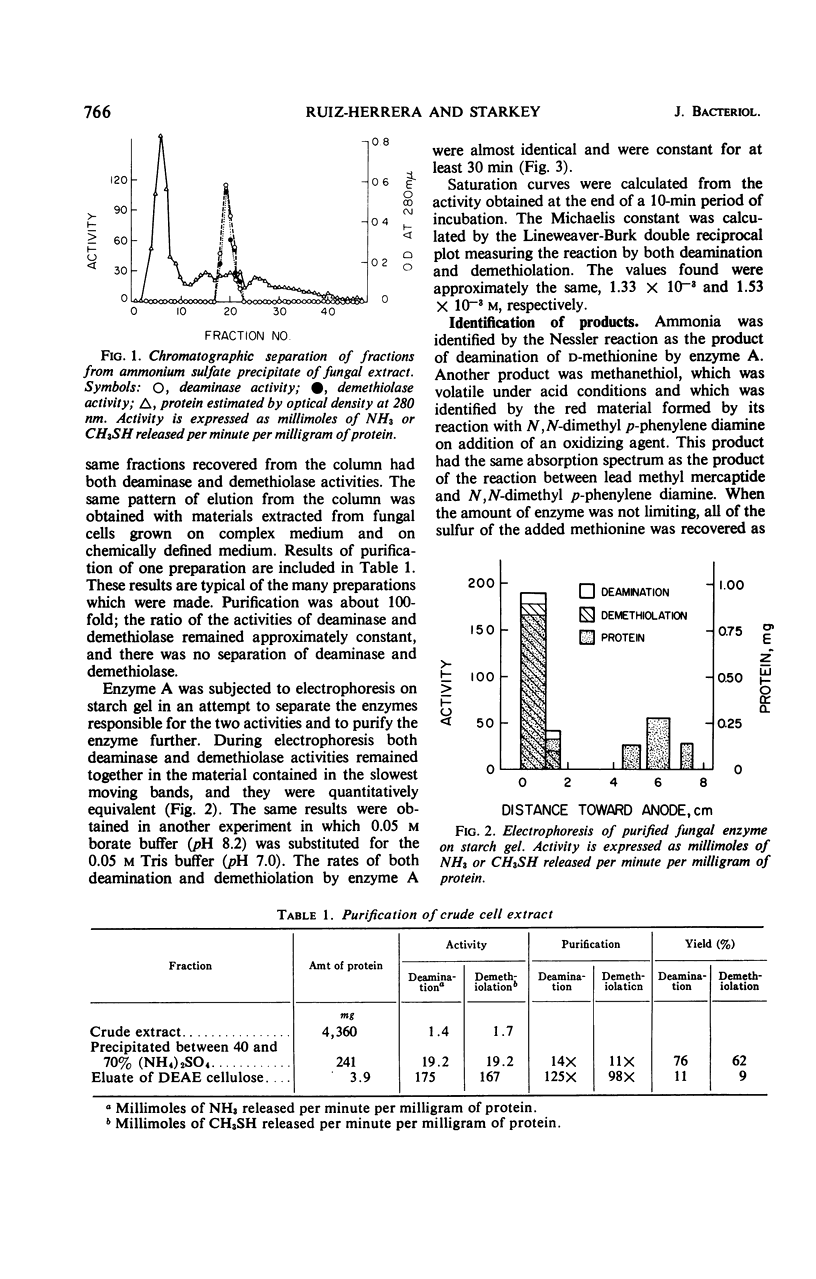
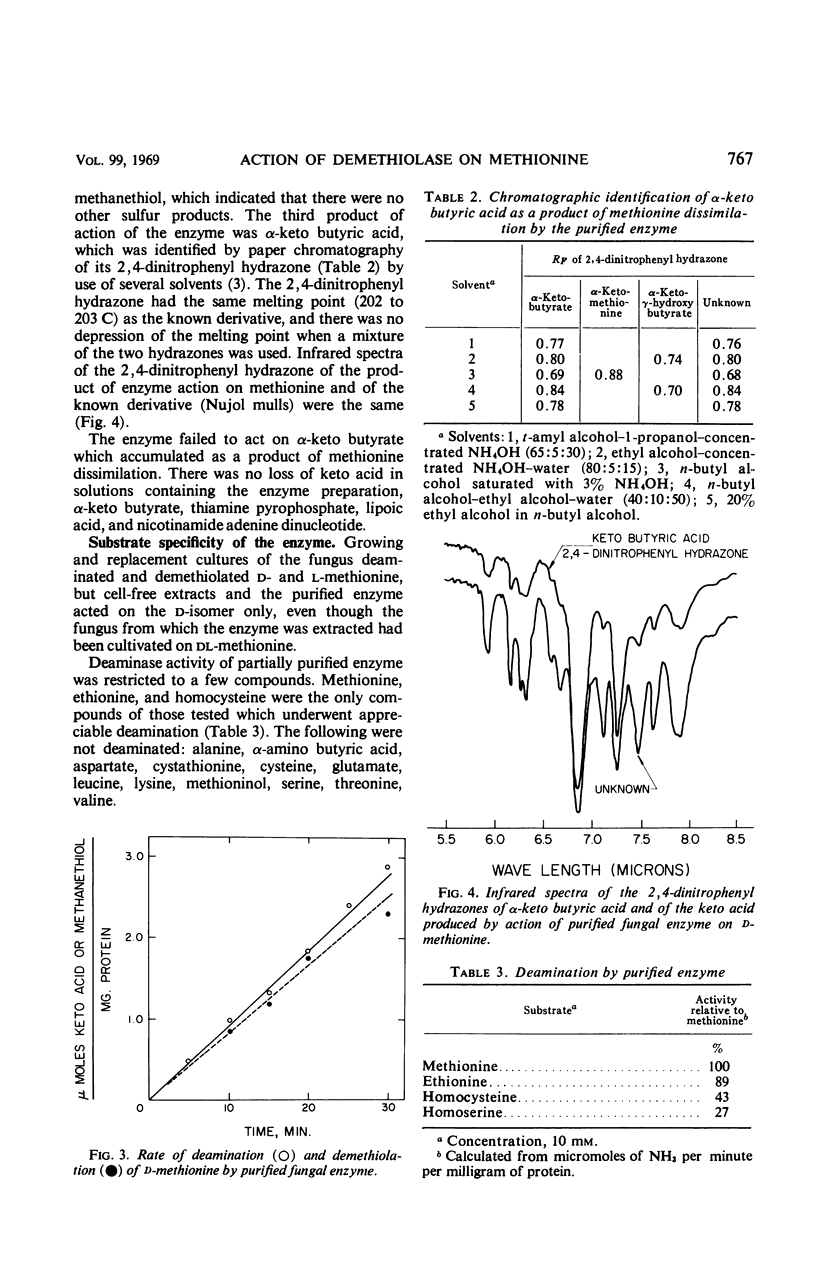
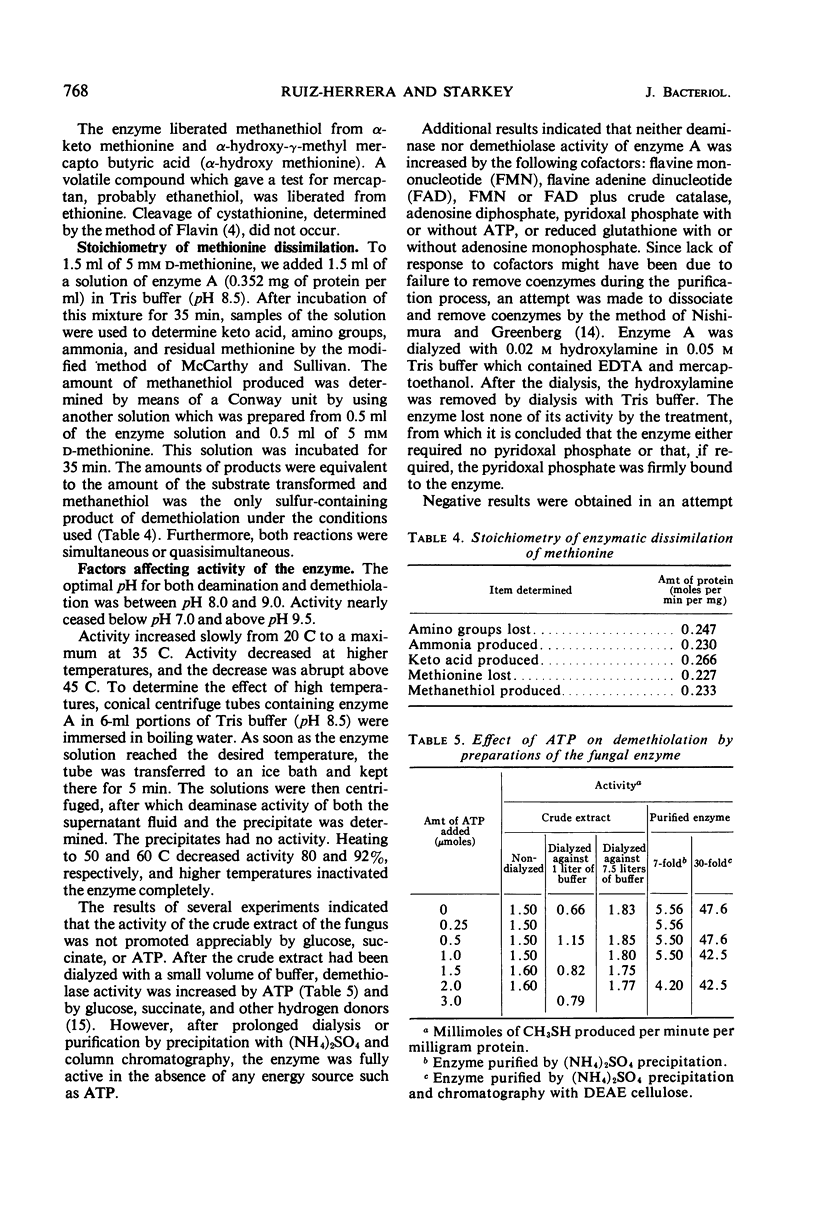
Enzyme preparations obtained from the mycelium of Aspergillus species broke down methionine by co-dissimilation. The deaminase and demethiolase activities of crude extracts were increased 100-fold by precipitation with (NH4)2SO4 and column chromatography on diethylaminoethyl cellulose. The enzyme acted on d-methionine but not on l-methionine. The enzyme was labile: it was inactivated by oxygen and ascorbic acid but ethylenediaminetetraacetic acid and mercaptoethanol preserved its activity. Enzyme activity decreased even at 4 and −30 C and was lost rapidly above 45 C. It was most rapid at 35 C and at pH 8.0 to 9.0. For the following reasons, it was concluded that deamination and demethiolation of methionine were effected by the same enzyme: both activities increased equally at each stage of purification; ammonia, methanethiol, and α-keto butyric acid were formed in amounts equivalent to the amount of methionine dissimilated; the Km and optimal pH for formation of both keto acid and methanethiol were the same; both activities remained in the same fractions that were separated by electrophoresis and the activities were equivalent. The purified enzyme demethiolated α-keto methionine and α-hydroxy methionine and split the sulfur linkage of ethionine but did not cleave cystathionine. Few amino acids were deaminated. The enzyme was sensitive to some carbonyl and sulfhydryl reagents and was relatively insensitive to heavy metals other than Hg++. The Km was 1.3 × 10−3 to 1.5 × 10−3m at pH 7.0. No requirement for cofactors was noted, and attempts to dissociate the enzyme, including dialysis with hydroxylamine, were unsuccessful.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACGEE J., DOUDOROFF M. A new phosphorylated intermediate in glucose oxidation. J Biol Chem. 1954 Oct;210(2):617–626. [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- MITSUHASHI S. Decomposition of thioether derivatives by bacteria; methylmercaptan formation and the properties of the responsible enzyme. Jpn J Exp Med. 1949 Sep;20(2):211–222. [PubMed] [Google Scholar]
- NISHIMURA J. S., GREENBERG D. M. Purification and properties of L-threonine dehydrase of sheep liver. J Biol Chem. 1961 Oct;236:2684–2691. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Starkey R. L. Dissimilation of methionine by fungi. J Bacteriol. 1969 Aug;99(2):544–551. doi: 10.1128/jb.99.2.544-551.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESENDANGER S., NISMAN B. La Lméthionine démercapto-désaminase: un nouvel enzyme à pyridoxal-phosphate. C R Hebd Seances Acad Sci. 1953 Oct 5;237(14):764–765. [PubMed] [Google Scholar]


