Abstract
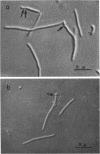
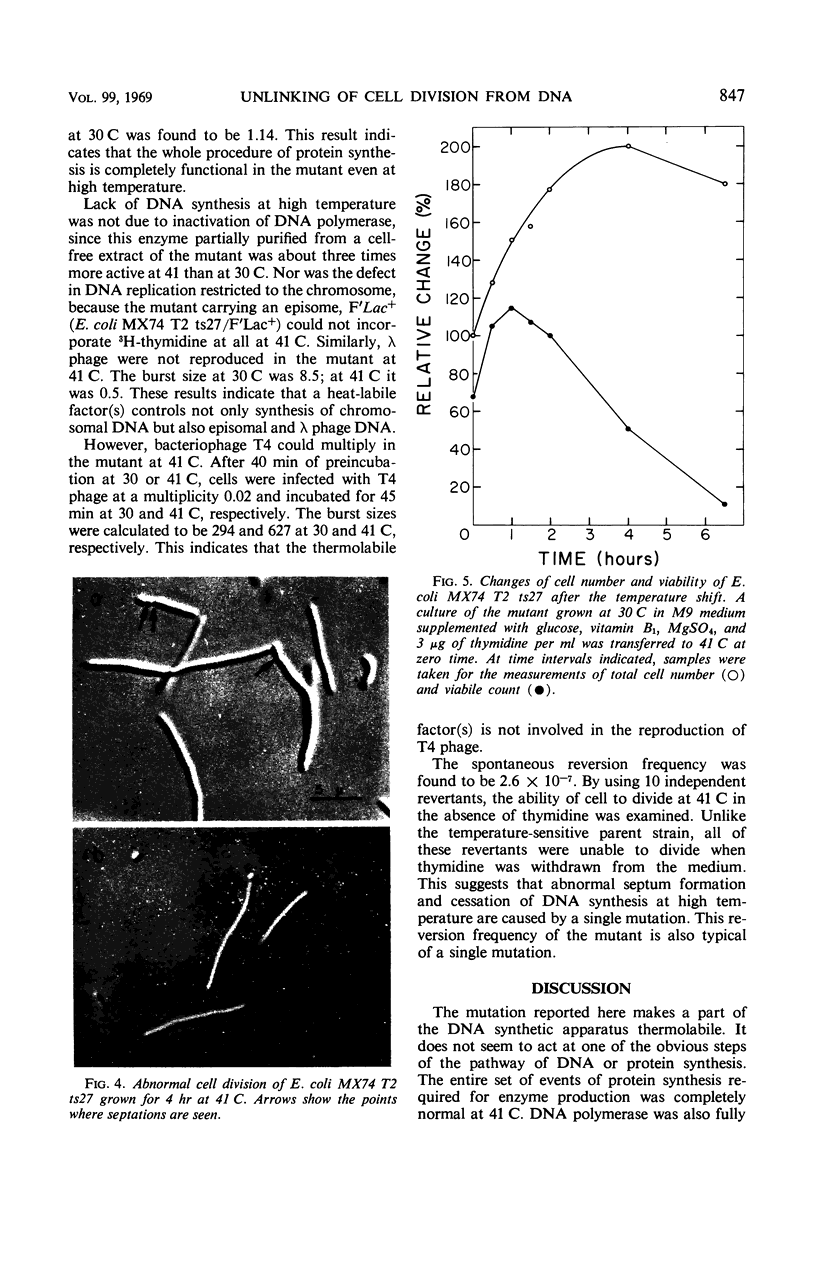
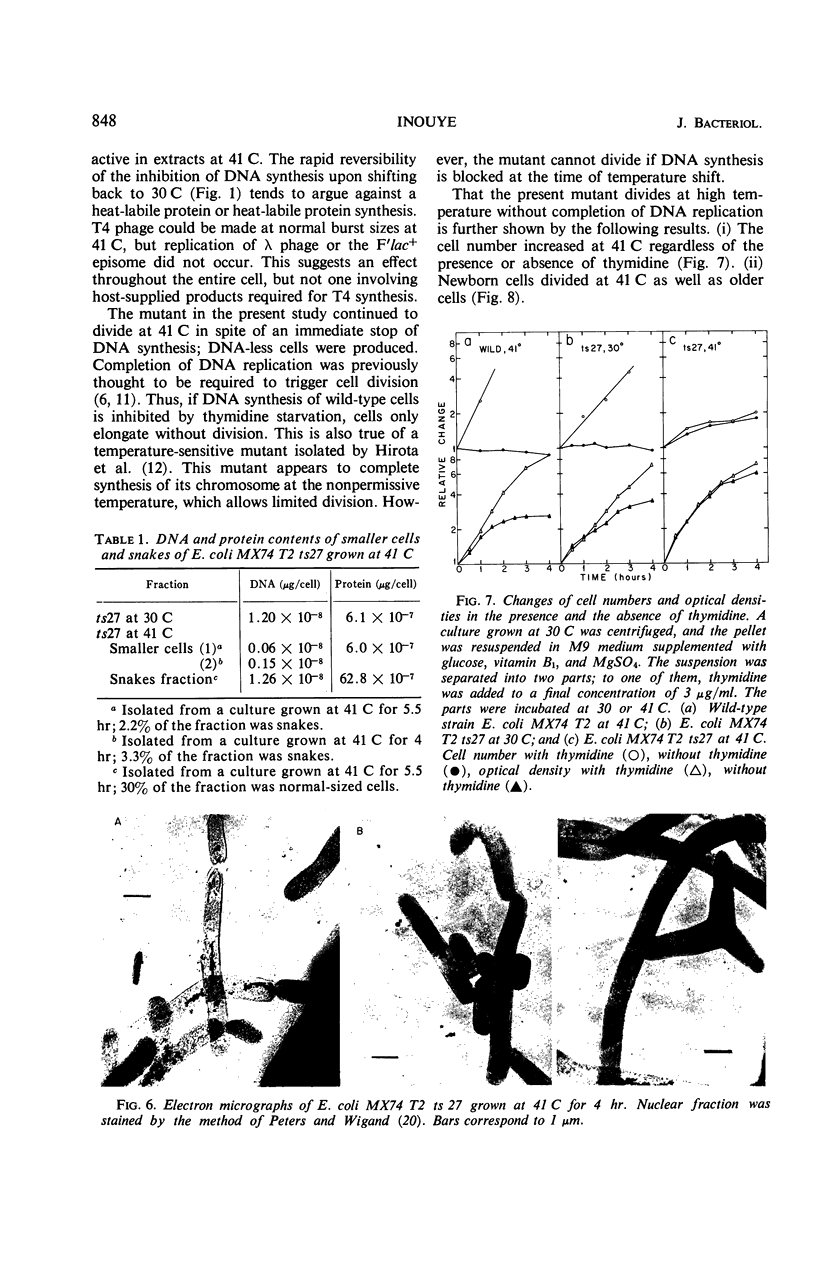
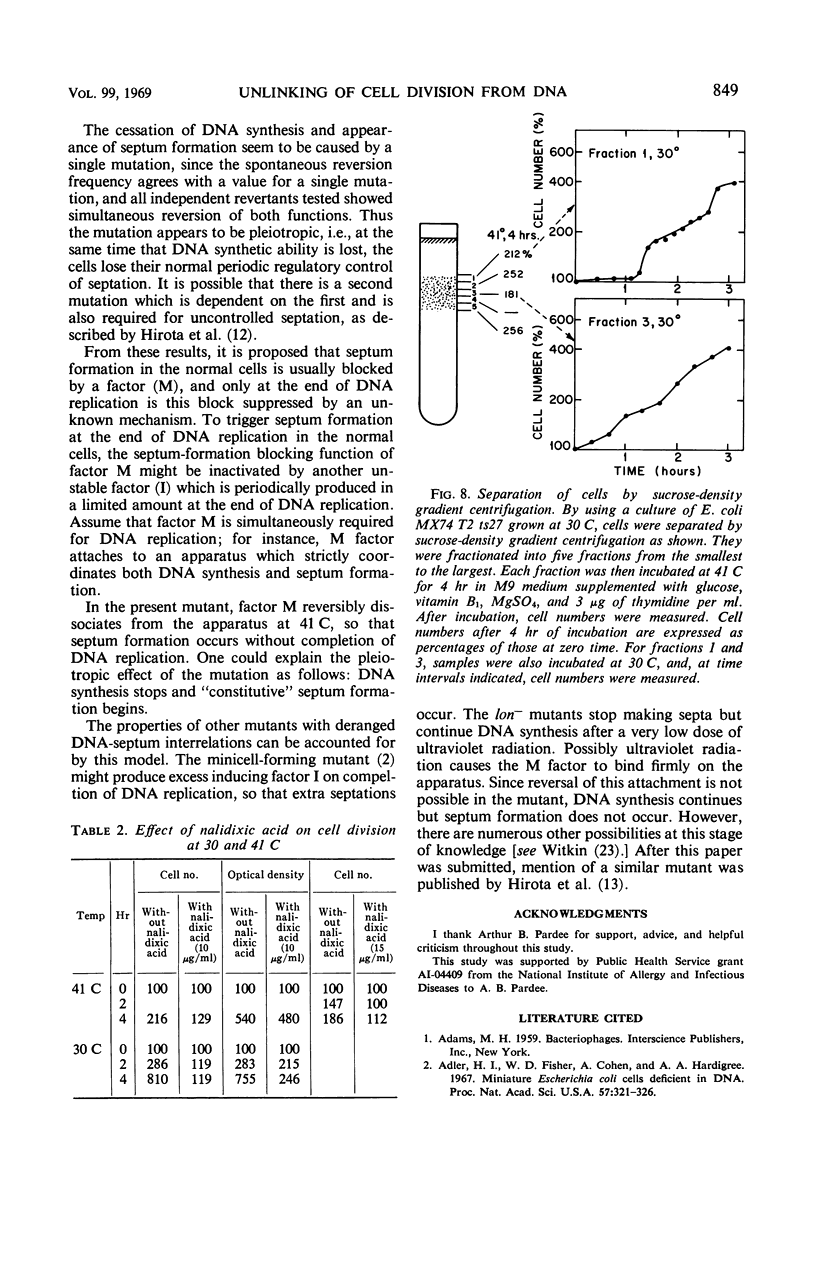
A new type of temperature-sensitive deoxyribonucleic acid (DNA) synthesis mutant, which can divide without a completion of DNA replication, was isolated from a thymidine-requiring Escherichia coli strain by means of photo-bromouracil selection after nitrosoguanidine mutagenesis. In this mutant, in spite of the fact that DNA synthesis stopped immediately after the temperature shift from 30 to 41 C, cells could continue to divide, though at a reduced rate. This cell division without DNA synthesis at 41 C is further supported by the following results. (i) Cell division took place at high temperature without addition of thymidine but not at all at 30 C. The parent strain of the mutant did not divide at 41 C without thymidine. (ii) Smaller cells isolated from the culture grown at 41 C did not contain DNA. This was shown by chemical analysis of the smaller cells and on electron micrographs. Ability of cells to divide was examined according to sizes of cells. By using the culture at 30 C, cells of various sizes were separated by means of sucrose-density gradient centrifugation. It was found that all cell fractions, including the smallest one, could divide at high temperature. These results suggest that in this mutant the completion of DNA replication is not required for triggering cell division at high temperature. Heat sensitivity of a factor which links cell division with DNA replication appears to be responsible. Some possible mechanisms of the coordination between cell division and DNA replication are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHOEFFER F., SCHALLER H. A METHOD FOR SELECTIVE ENRICHMENT OF MUTANTS BASED ON THE HIGH UV SENSITIVITY OF DNA CONTAINING 5-BROMOURACIL. Biochem Biophys Res Commun. 1965 Jun 18;20:93–97. [PubMed] [Google Scholar]
- Bonhoeffer F. DNA transfer and DNA synthesis during bacterial conjugation. Z Vererbungsl. 1966;98(2):141–149. doi: 10.1007/BF00897186. [DOI] [PubMed] [Google Scholar]
- Boyle J. V., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. Vi. Cell-free studies. J Bacteriol. 1969 Jan;97(1):230–236. doi: 10.1128/jb.97.1.230-236.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Maas W. K. Inhibition of replication of an F'lac episome in Hfr cells of Escherichia coli. J Bacteriol. 1968 Feb;95(2):531–539. doi: 10.1128/jb.95.2.531-539.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Characterization of two bacterial mutants with temperature-sensitive synthesis of DNA. Genetics. 1968 Sep;60(1):1–17. doi: 10.1093/genetics/60.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Jacob F., Ryter A., Buttin G., Nakai T. On the process of cellular division in Escherichia coli. I. Asymmetrical cell division and production of deoxyribonucleic acid-less bacteria. J Mol Biol. 1968 Jul 14;35(1):175–192. doi: 10.1016/s0022-2836(68)80046-4. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Gross J. D. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J Bacteriol. 1967 Nov;94(5):1603–1608. doi: 10.1128/jb.94.5.1603-1608.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. A SECOND PERMEASE FOR METHYL-THIO-BETA-D-GALACTOSIDE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:591–593. doi: 10.1016/0304-4165(65)90029-2. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pizer L. I. Abortive infection of Escherichia coli strain W by T2 bacteriophage. J Mol Biol. 1968 Oct 14;37(1):131–149. doi: 10.1016/0022-2836(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]