Abstract
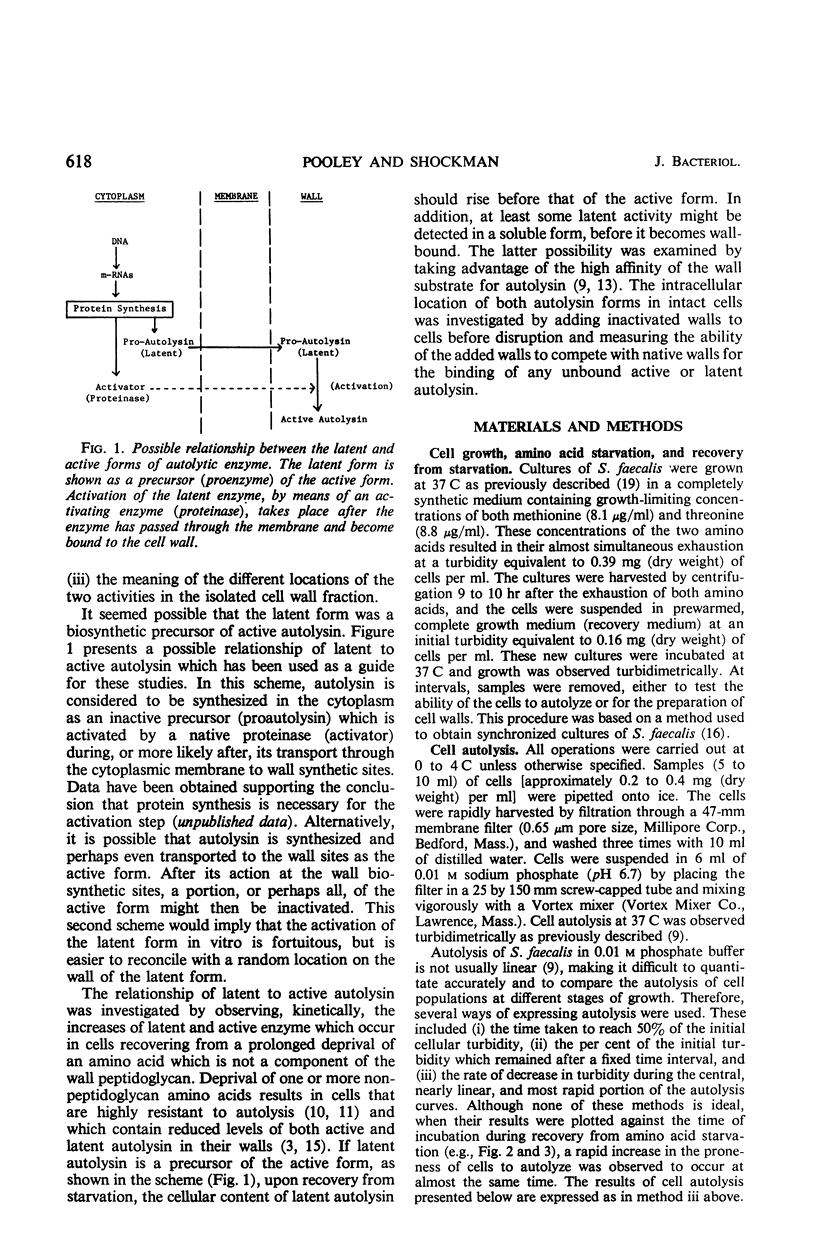
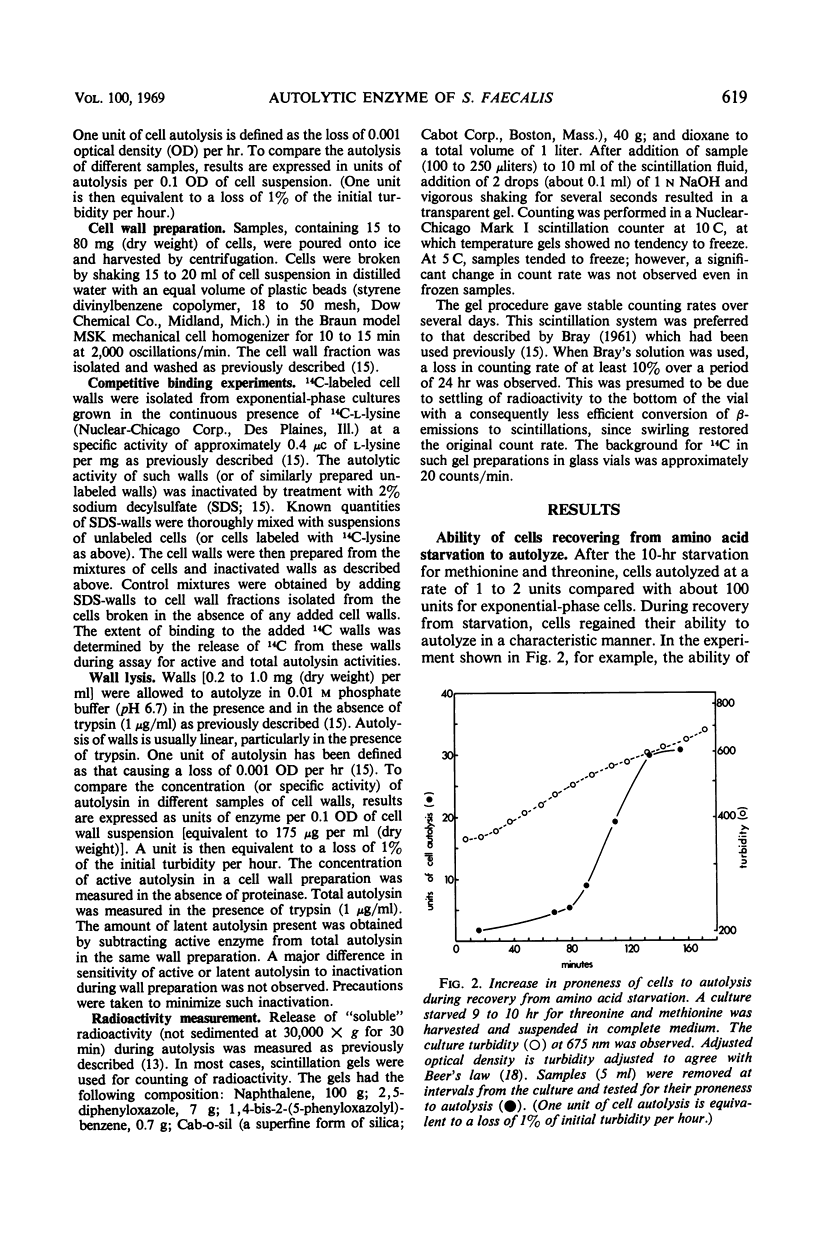
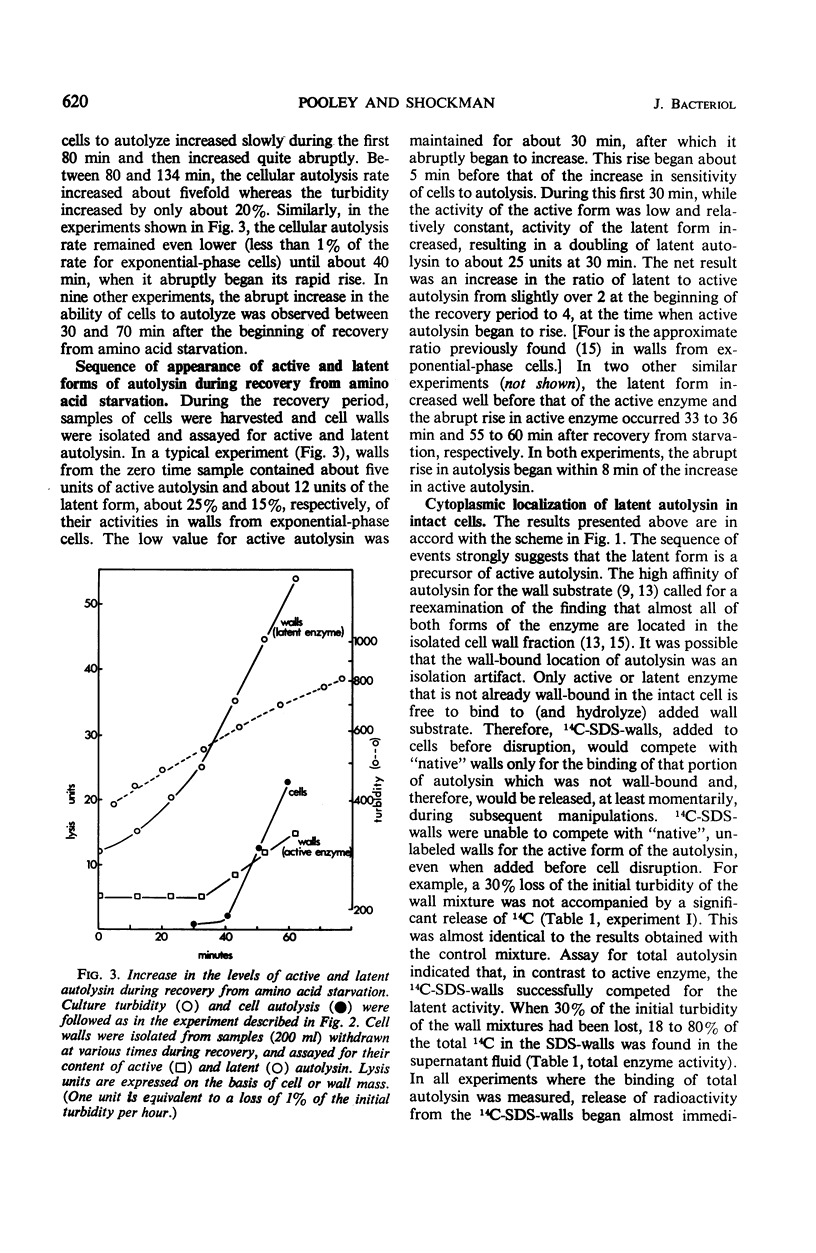
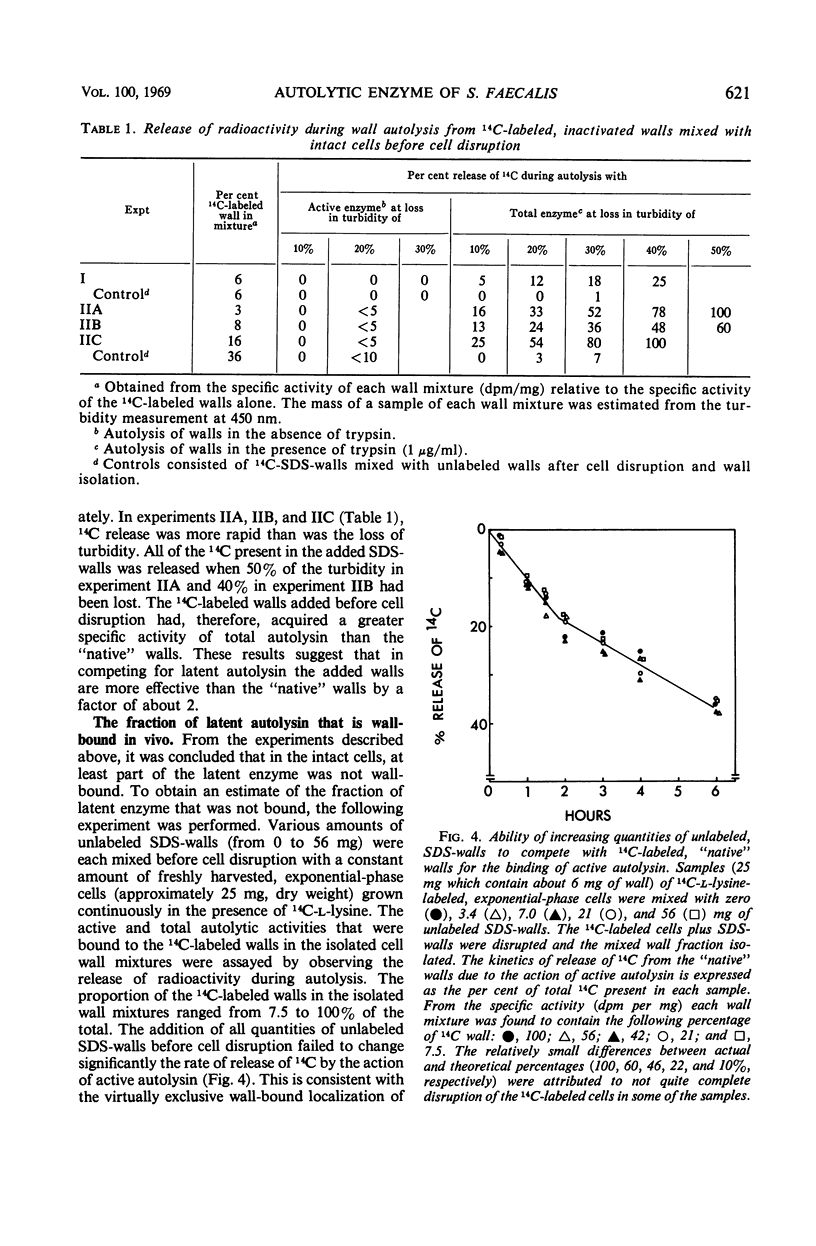
A 10-hr starvation of Streptococcus faecalis ATCC 9790 for the amino acids methionine and threonine results in cells which are resistant to autolysis and which contain greatly reduced quantities of both active and latent (proteinase activable) forms of the autolytic enzyme (an N-acetyl-muramide glycanhydrolase). Cell walls were isolated from cells harvested at various times during the recovery from such starvation and were assayed for active and latent forms of the autolysin. Within 10 min of recovery the latent enzyme began to increase. Only after 30 to 60 min did the active enzyme begin to increase; after a similar lag, the cells' proneness to lysis markedly increased. The intracellular localization of both forms of the autolysin was examined, using as an experimental tool the ability of added cell wall to bind autolysin. 14C-lysine-labeled, inactivated cell walls were added to exponential-phase cells, which were then disrupted, and the mixed wall population was isolated. Measurement of the 14C release during wall autolysis indicated that the active enzyme in the cells was not available for binding to the added 14C-labeled walls and was therefore wall-bound in vivo. In contrast, up to 85% of latent autolysin activity was found to have been efficiently bound to the added 14C walls. The results obtained suggest (i) cellular autolysis is a reflection of the level of active enzyme and not of latent enzyme, and (ii) autolysin is synthesized and mainly located in the cytoplasm as an inactive latent precursor (proenzyme) which is transported to sites on the cell wall associated with wall biosynthesis, where it becomes activated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilezikian J. P., Kaempfer R. O., Magasanik B. Mechanism of tryptophanase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):495–506. doi: 10.1016/0022-2836(67)90054-x. [DOI] [PubMed] [Google Scholar]
- COLE R. M., HAHN J. J. Cell wall replication in Streptococcus pyogenes. Science. 1962 Mar 2;135(3505):722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- Conover M. J., Thompson J. S., Shockman G. D. Autolytic enzyme of Streptococcus faecalis: release of soluble enzyme from cell walls. Biochem Biophys Res Commun. 1966 Jun 13;23(5):713–719. doi: 10.1016/0006-291x(66)90459-1. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. ACTIVATION OF STREPTOCOCCAL PROTEINASE AND ITS ZYMOGEN BY BACTERIAL CELL WALLS. Nature. 1965 Apr 3;206:33–34. doi: 10.1038/206033a0. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- Schwarz U., Leutgeb W. In vivo studies on murein synthesis. Folia Microbiol (Praha) 1967;12(3):279–282. doi: 10.1007/BF02868744. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Martin J. T. Autolytic enzyme system of Streptococcus faecalis. IV. Electron microscopic observations of autolysin and lysozyme action. J Bacteriol. 1968 Nov;96(5):1803–1810. doi: 10.1128/jb.96.5.1803-1810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Stonehill E. H., Hutchison D. J. Chromosomal mapping by means of mutational induction in synchronous populations of Streptococcus faecalis. J Bacteriol. 1966 Jul;92(1):136–143. doi: 10.1128/jb.92.1.136-143.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]


