Abstract
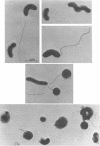
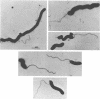
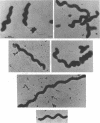
A reliable method has been developed for the isolation of host-independent (H-I; i.e., “saprophytic”) strains of Bdellovibrio from host-dependent (H-D; i.e., “parasitic”) cultures. The technique involves growing streptomycin-resistant (Smr) H-D cultures on streptomycin-susceptible (Sm8) host cells. A lysate containing large numbers of the Smr H-D cells and some remaining Sm8 host cells is transferred to a selection medium which contains the antibiotic. The Sm8 host cells in the lysate are killed, and the Smr H-I strains develop in broth within 3 to 6 days. By use of this method, it has been possible to isolate H-I strains from 16 different H-D Bdellovibrio strains studied. The frequency of occurrence of host independence is in the range of one H-I colony per 106 to 107 plaque-forming units of H-D bdellovibrios. The H-I cultures are nonfermentative, do not reduce nitrate, are strongly proteolytic, are oxidase-positive, and do not utilize 14 different carbon compounds as sources of energy for growth. Most H-I cultures are catalase-positive upon initial isolation from H-D lysates, but some cultures lose this enzyme upon subsequent transfers through host-free media. Most H-I bdellovibrios are pleomorphic, consisting of vibrio- to spiral-shaped cells typically measuring 0.3 to 0.4 μm in width and 1 to 10 μm in length. All H-I bdellovibrios have a cytochrome a and c component (H-I A3.12 differs from the other strains in the location of the peaks of the cytochrome spectrum). All are sensitive to oxytetracycline and (except for strain H-I A3.12) to the vibriostatic pteridine 0/129; most bdellovibrios, except for H-I A3.12, are generally uniformly resistant or susceptible to a given antibiotic. Bdellovibrio and Vibrio spp. have common cytochrome difference spectra and susceptibilities to oxytetracycline and to the vibriostatic pteridine 0/129. All H-I bdellovibrios examined produce an exocellular protease which digests heat-killed host cells. Bdellovibrios possessing predatory and bacteriolytic properties could be reselected from H-I bdellovibrio cultures growing in the presence of living host cells. Attempts to select for bacteriolytic isolates from Vibrio and Spirillum spp. were unsuccessul.
Full text
PDF






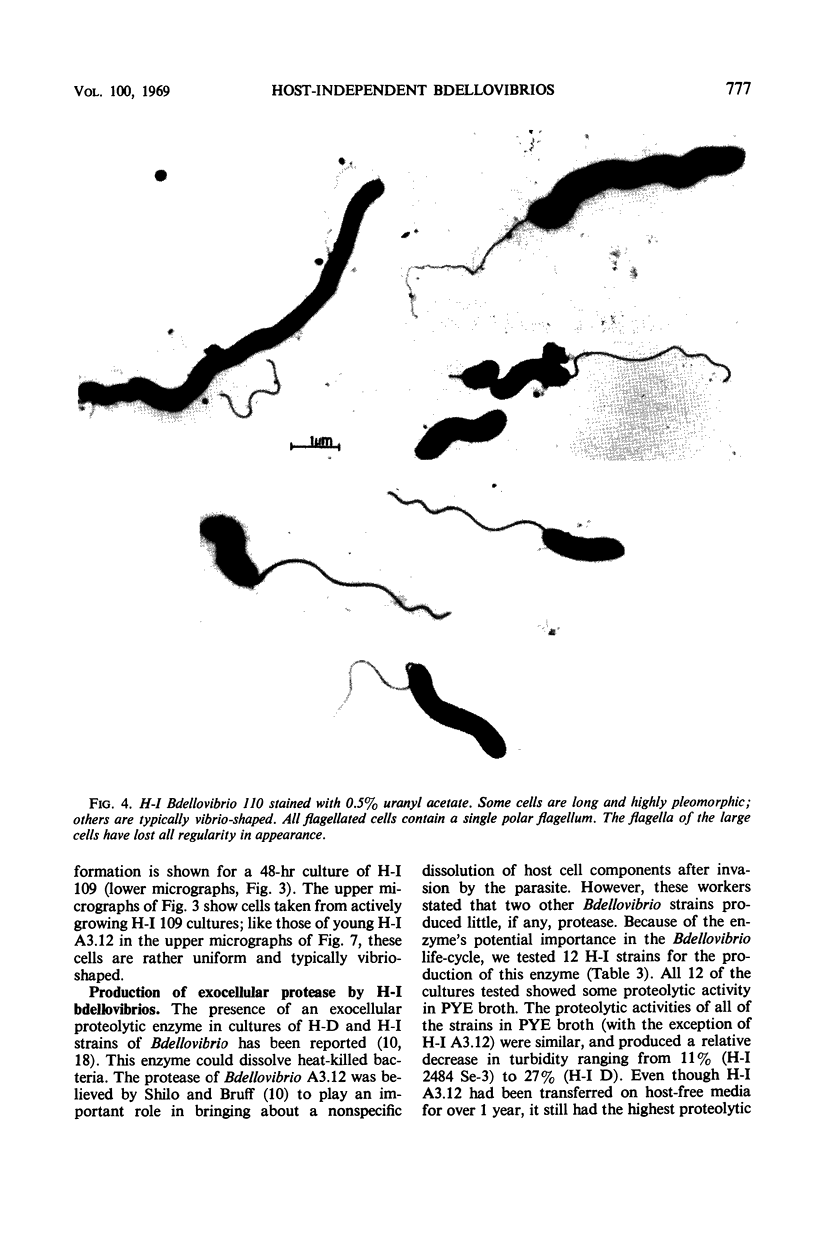
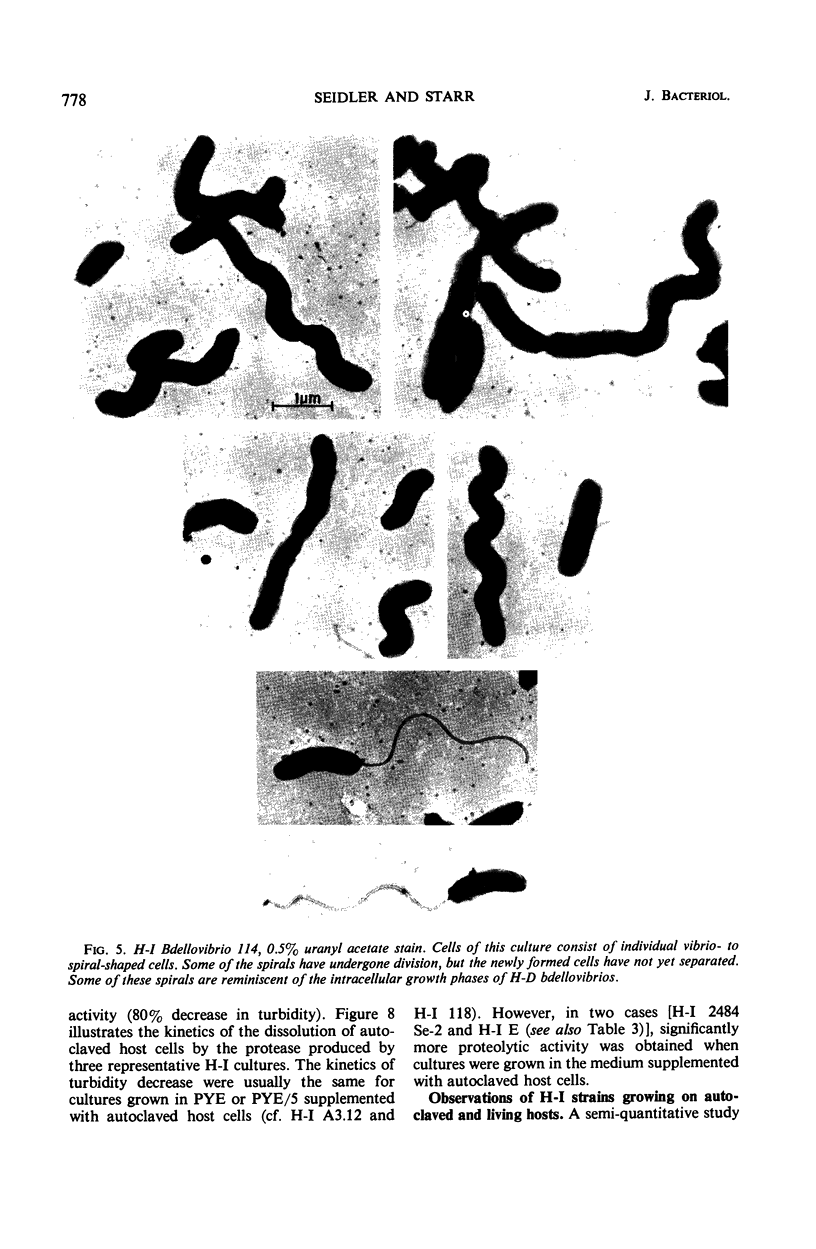
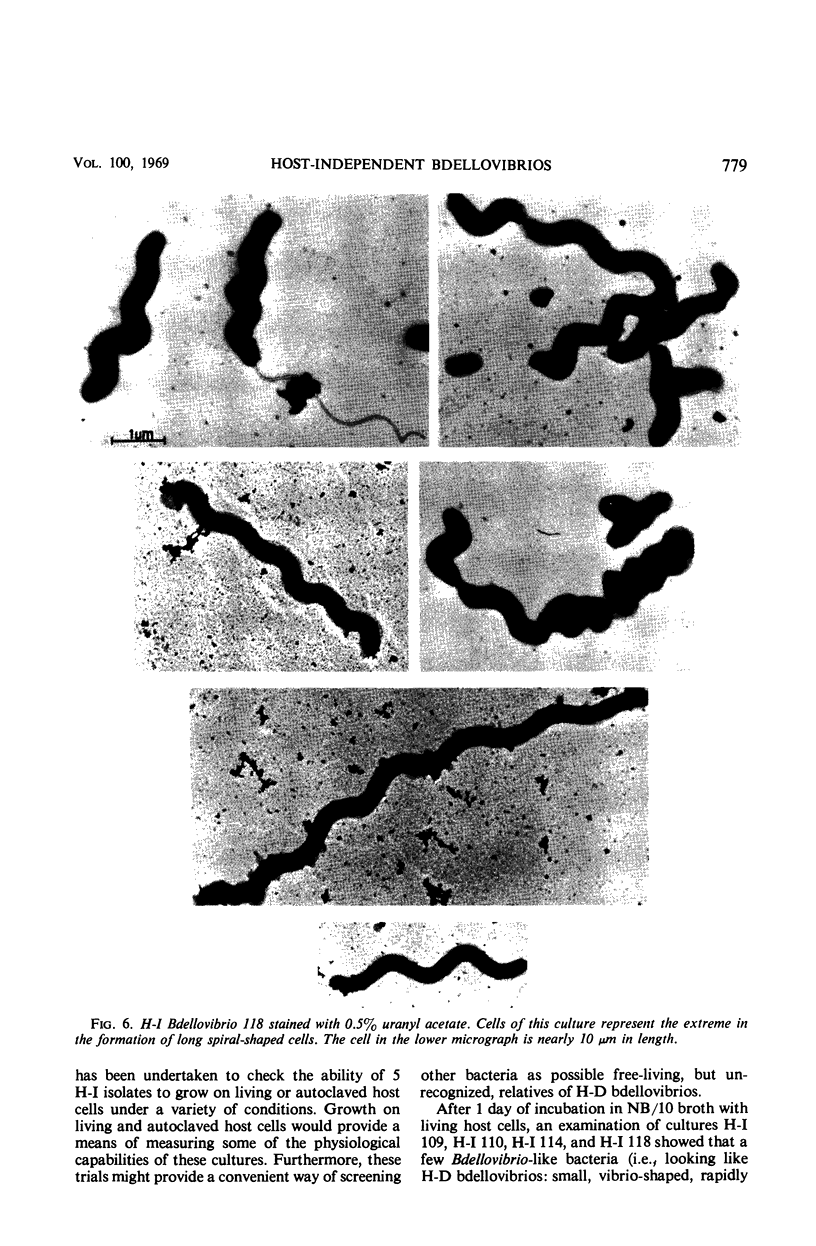
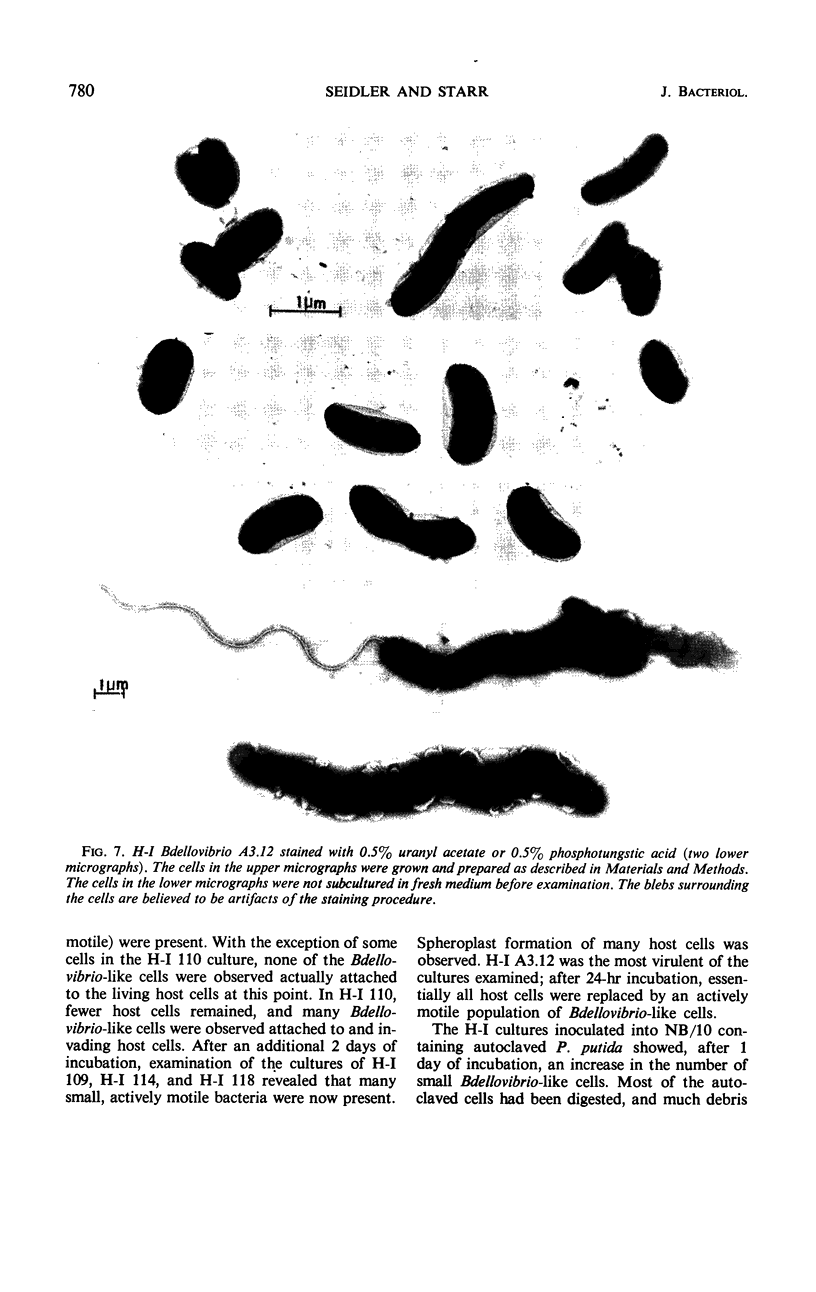
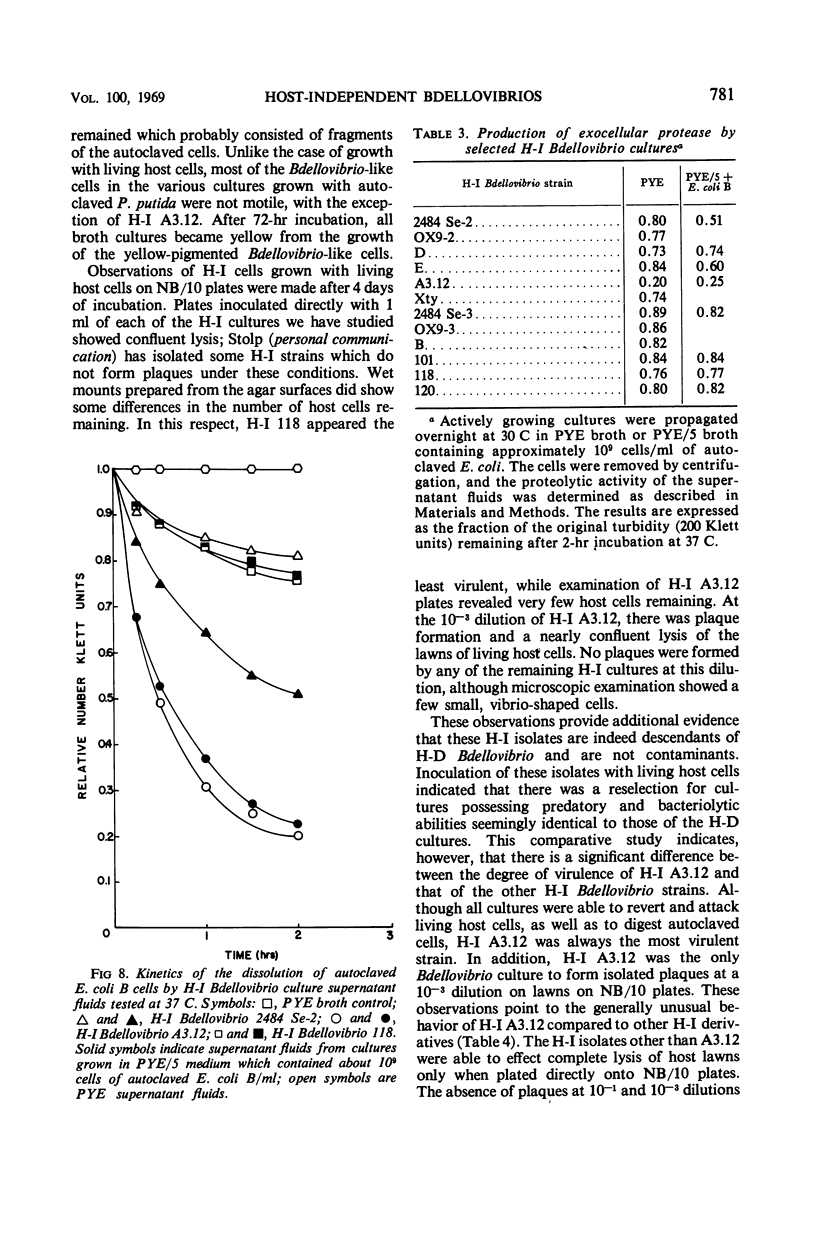
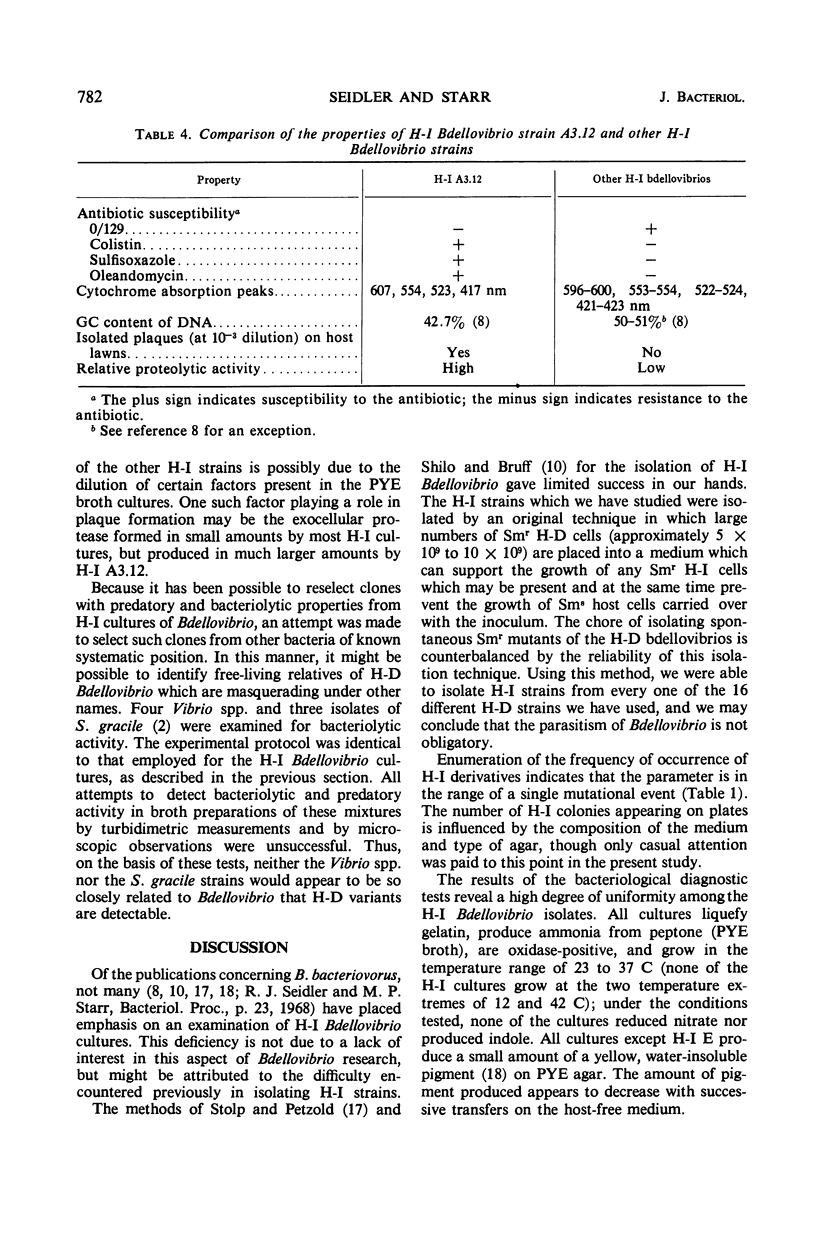



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger A., Drews G., Ladwig R. Wirtskreis und Infektionscyclus eines neu isolierten Bdellovibrio bacteriovorus-Stammes. Arch Mikrobiol. 1968 May 8;61(3):261–279. [PubMed] [Google Scholar]
- Canale-Parola E., Rosenthal S. L., Kupfer D. G. Morphological and physiological characteristics of Spirillum gracile sp. n. Antonie Van Leeuwenhoek. 1966;32(2):113–124. doi: 10.1007/BF02097451. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., KERRIDGE D., HORNE R. W. THE FINE STRUCTURE AND MODE OF ATTACHMENT OF THE SHEATHED FLAGELLUM OF VIBRIO METCHNIKOVII. J Cell Biol. 1963 Aug;18:327–336. doi: 10.1083/jcb.18.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lépine P., Guélin A., Sisman J., Lamblin D. Etude au microscope électronique de la lyse de Salmonella par Bdellovibrio bacteriovorus. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jun 19;264(25):2957–2960. [PubMed] [Google Scholar]
- SEBALD M., VERON M. TENEUR EN BASES DE L'ADN ET CLASSIFICATION DES VIBRIONS. Ann Inst Pasteur (Paris) 1963 Nov;105:897–910. [PubMed] [Google Scholar]
- SHEWAN J. M., HODGKISS W. A method for the rapid differentiation of certain nonpathogenic, asporogenous bacilli. Nature. 1954 Jan 30;173(4396):208–209. doi: 10.1038/173208b0. [DOI] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Factors affecting the intracellular parasitic growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J Bacteriol. 1969 Feb;97(2):912–923. doi: 10.1128/jb.97.2.912-923.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P., Mandel M. Deoxyribonucleic acid characterization of Bdellovibrios. J Bacteriol. 1969 Nov;100(2):786–790. doi: 10.1128/jb.100.2.786-790.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Structure of the flagellum of Bdellovibrio bacteriovorus. J Bacteriol. 1968 May;95(5):1952–1955. doi: 10.1128/jb.95.5.1952-1955.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Simpson F. J., Robinson J. Some energy-producing systems in Bdellovibrio bacteriovorus, strain 6-5-S. Can J Biochem. 1968 Aug;46(8):865–873. doi: 10.1139/o68-129. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Baigent N. L. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J Bacteriol. 1966 May;91(5):2006–2017. doi: 10.1128/jb.91.5.2006-2017.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron M. Taxonomie numériques de vibrions et de certaines bactéries comparables. II. Corrélation entre les similitudes phénétiques et la composition en bases de l'ADN. Ann Inst Pasteur (Paris) 1966 Dec;111(6):671–709. [PubMed] [Google Scholar]