Abstract
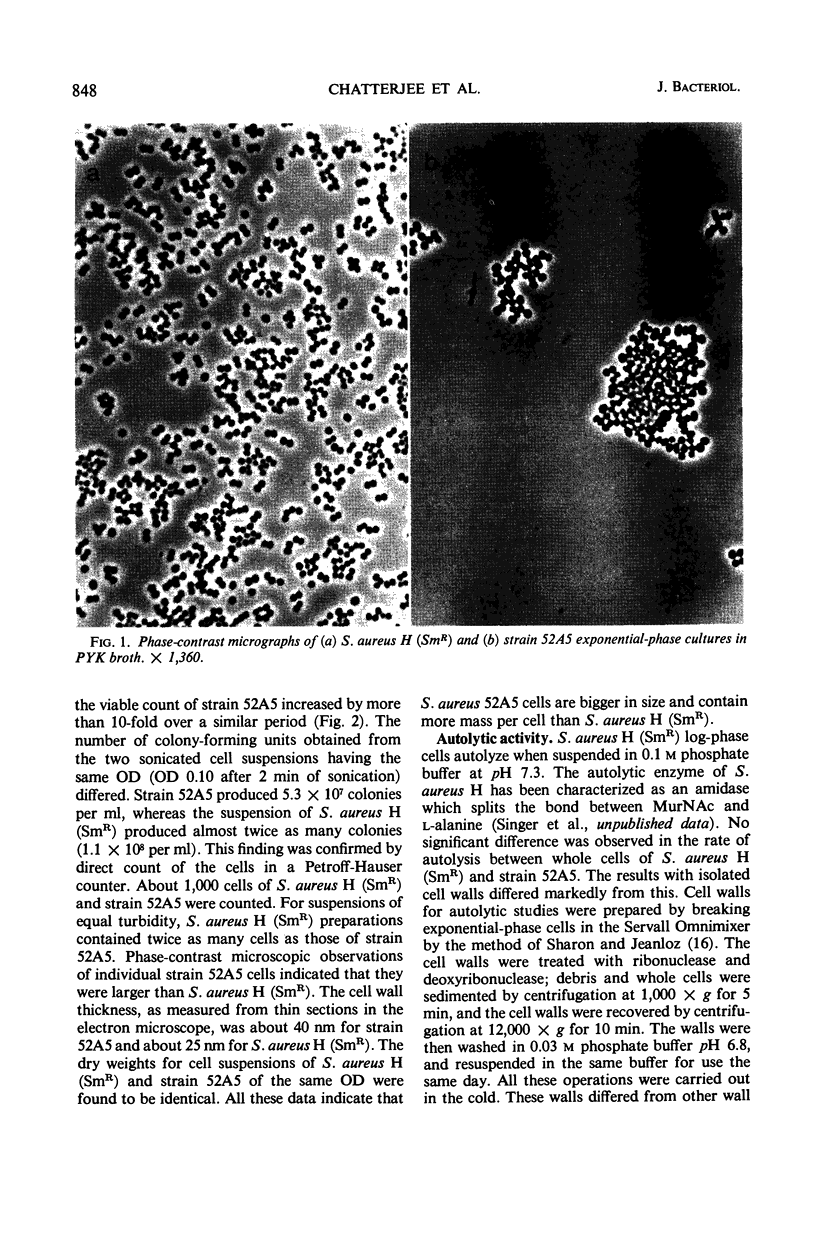
A phage-resistant mutant of Staphylococcus aureus H (SmR), S. aureus 52A5, was previously shown to lack polymeric teichoic acid. This paper characterizes other phenotypic differences between the strains. In broth cultures the mutant cells grew more slowly, were larger, and formed much larger clumps than the parent strain. The clumps of cells appeared to be covalently linked and could only be separated by mild sonic energy—a process which yielded viable cells. Mutant and parent cells autolyzed at equal rates, whereas isolated cell walls of the mutant strain autolyzed faster than the wild type. Nevertheless, the specific activity of the autolytic enzyme in the wild type soluble fraction was much higher than in the mutant. In contrast to the parent, strain 52A5 failed to accumulate nucleotide-bound murein precursors when treated with penicillin. Mutant strains with these characteristics were repeatedly isolated both spontaneously and by chemical mutagenesis. Strain 52A5 was shown to be fully revertible. Thus, it appears to be a pleiotropic mutation, and the possible nature of the defect which causes these varied effects is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Ionesco H., Schaeffer P. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta. 1966 Aug 24;124(2):415–417. doi: 10.1016/0304-4165(66)90211-x. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D., Strominger J. L. Structure of the cell wall of Staphylococcus aureus. 8. Structure and chemical synthesis of the basic peptides released by the Myxobacterium enzyme. Biochemistry. 1967 Aug;6(8):2591–2598. doi: 10.1021/bi00860a042. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. II. Isolation from Staphylococcus aureus. J Biol Chem. 1952 Feb;194(2):877–884. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- ROSENTHAL S., SHARON N. THE USE OF SEPHADEX G-25 FOR THE ISOLATION OF NUCLEOTIDE SUGAR DERIVATIVES FROM MICROCOCCUS LYSODEIKTICUS. Biochim Biophys Acta. 1964 Nov 1;83:378–380. doi: 10.1016/0926-6526(64)90025-4. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Sharon N., Jeanloz R. W. A procedure for the preparation of gram-quantities of bacterial cell walls. Experientia. 1964 May 15;20(5):253–254. doi: 10.1007/BF02151786. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Woodward D. O., Munkres K. D. Alterations of a maternally inherited mitochondrial structural protein in respiratory-deficient strains of Neurospora. Proc Natl Acad Sci U S A. 1966 Apr;55(4):872–880. doi: 10.1073/pnas.55.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]



