Abstract
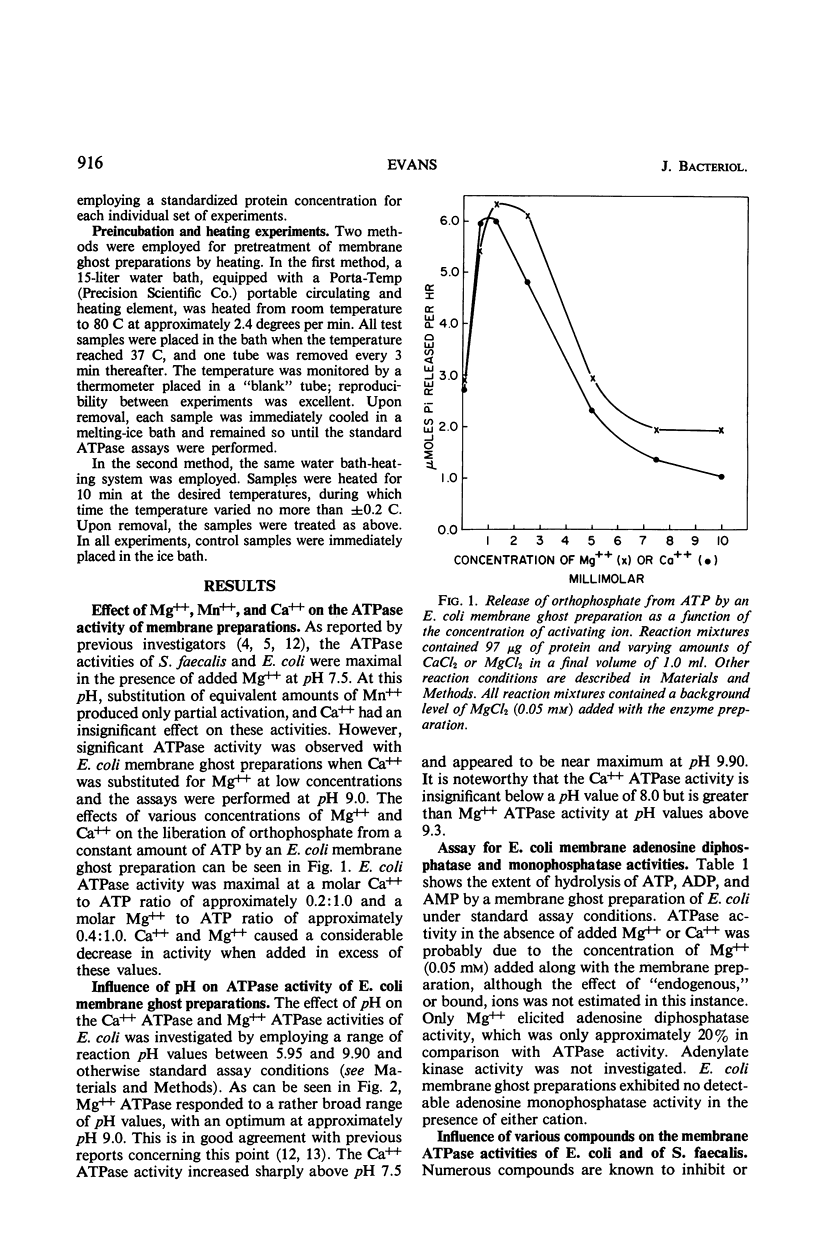
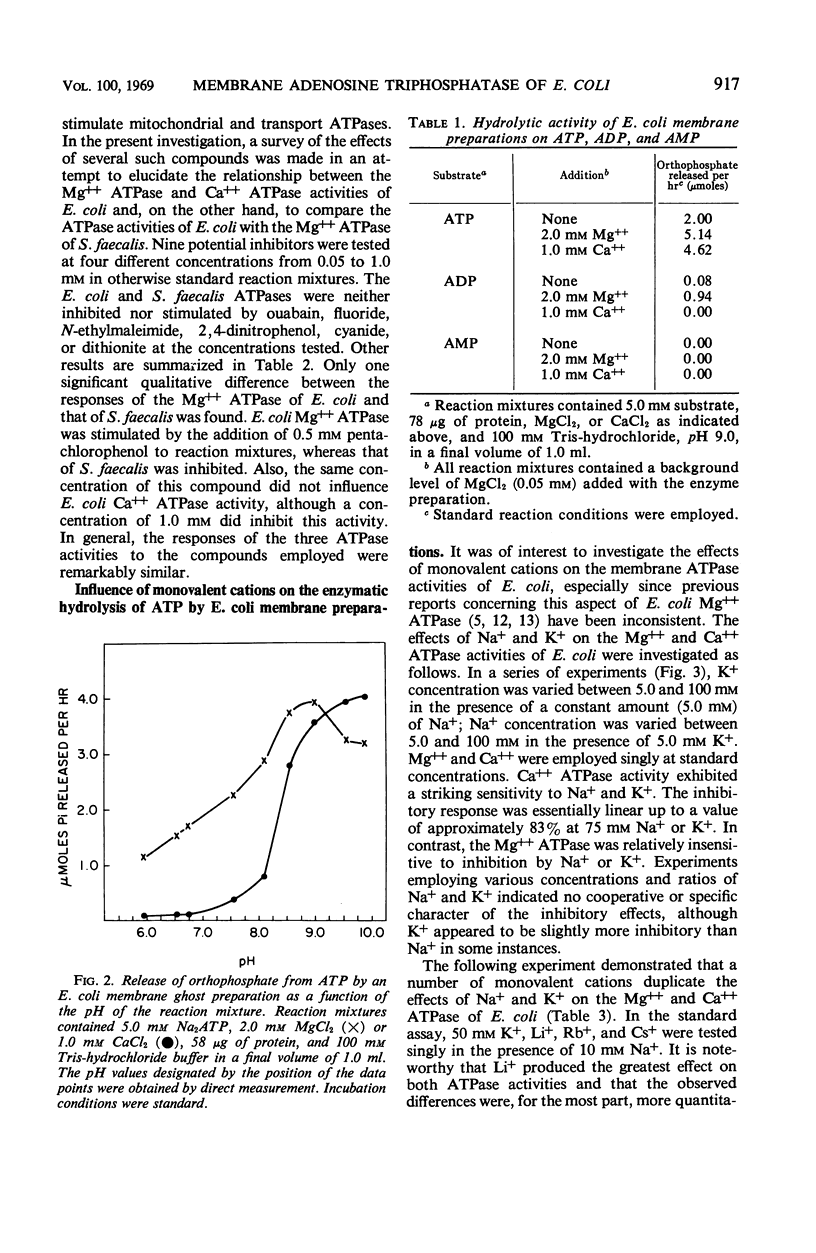
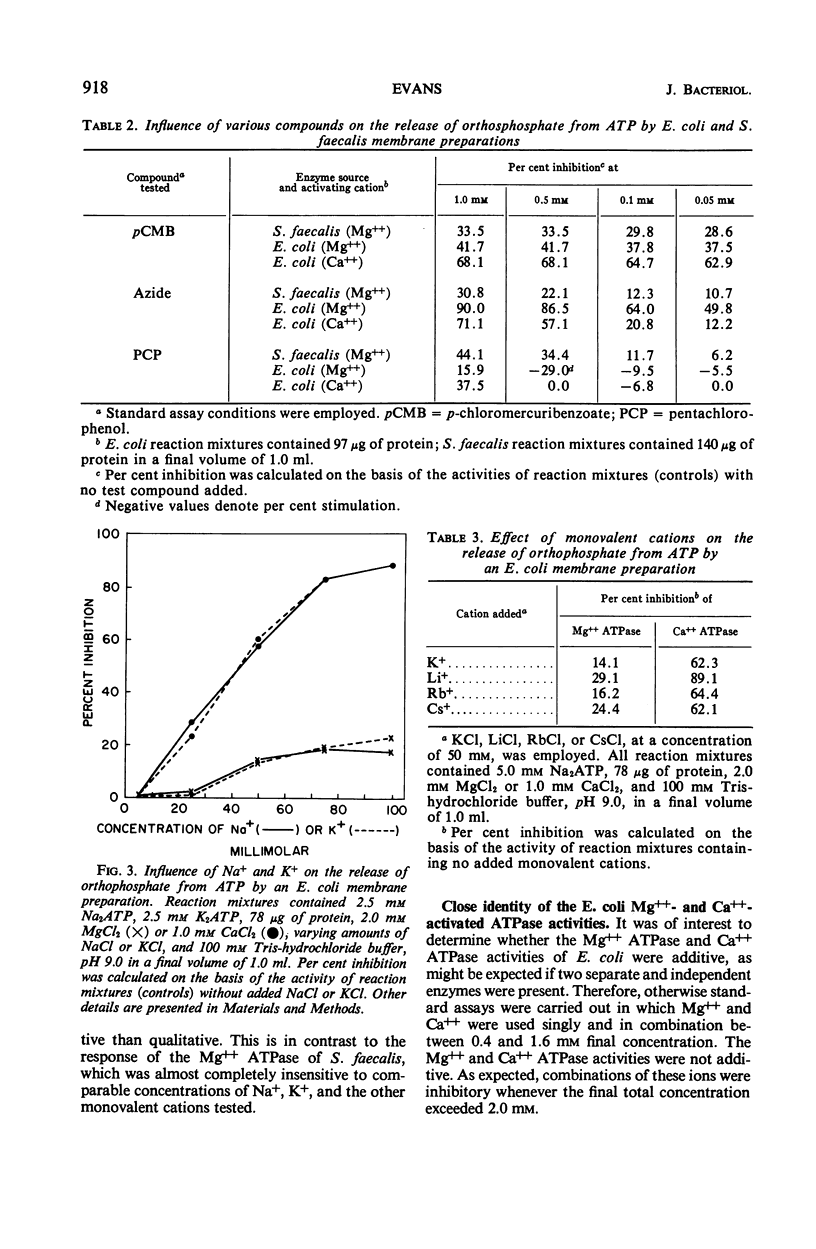
Membrane ghost preparations of Escherichia coli K-12 obtained by osmotic lysis of lysozyme-induced spheroplasts were found to possess both Mg++- and Ca++-activated adenosine 5′-triphosphatase (ATPase, EC 3.6.1.3) activities. Maximal activities of 1.0 to 1.5 μmoles of orthophosphate released per min per mg of protein were obtained at pH 9.0 with a molar Mg++ to adenosine 5′triphosphate (ATP) ratio of 2:5 and at pH 9.9 with a molar Ca++ to ATP ratio of 1:5. These ATPase activities were not altered by ouabain, fluoride, N-ethylmaleimide, 2,4-dinitrophenol, cyanide, or dithionite, but were inhibited by low concentrations of azide, p-chloromercuribenzoate, and pentachlorophenol. Mg++ ATPase was more susceptible to inhibition by azide than was Ca++ ATPase. Fifty per cent inactivation of both activities was observed when membrane ghost preparations were preincubated at 66 C for 10 min. The Mg++ and Ca++ ATPase activities of these preparations were not additive, but did respond independently to inhibition by monovalent cations. Ca++ ATPase was found to be very sensitive to inhibition by K+, Na+, Li+, Rb+, and Cs+; Mg++ ATPase was relatively insensitive to these ions. One possible interpretation of the results presented in this paper is that the membrane of E. coli possesses an ATPase which is activated by either Mg++ or Ca++ and that activation by Ca++ increases the susceptibility of this enzyme to inhibition by monovalent cations. Increased susceptibility of E. coli membrane ATPase to inhibition by monovalent cations such as Na+ and K+ as a consequence of Ca++ activation could represent a regulatory mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- Abrams A., Baron C. Reversible attachment of adenosine triphosphatase to streptococcal membranes and the effect of magnesium ions. Biochemistry. 1968 Feb;7(2):501–507. doi: 10.1021/bi00842a003. [DOI] [PubMed] [Google Scholar]
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide-adenine dinucleotide oxidation in Escherichia coli particles. 3. Cellular location of menadione reductase and ATPase activities. Can J Biochem. 1967 Jul;45(7):1107–1124. doi: 10.1139/o67-128. [DOI] [PubMed] [Google Scholar]
- Burnham J. C., Hageage G. J., Jr Adenosine phosphate hydrolases in cell fractions of Vitreoscilla. J Bacteriol. 1967 Jan;93(1):191–198. doi: 10.1128/jb.93.1.191-198.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Hughes D. E. The enzymic activity of the outer shell of Lactobacillus arabinosus. J Gen Microbiol. 1965 Jul;40(1):81–95. doi: 10.1099/00221287-40-1-81. [DOI] [PubMed] [Google Scholar]
- DRAPEAU G. R., MACLEOD R. A. NUTRITION AND METABOLISM OF MARINE BACTERIA. XII. ION ACTIVATION OF ADENOSINE TRIPHOSPHATASE IN MEMBRANES OF MARINE BACTERIAL CELLS. J Bacteriol. 1963 Jun;85:1413–1419. doi: 10.1128/jb.85.6.1413-1419.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWALT J. W., WEIBULL C., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. II. Stimulation and inhibition of the enzymic activities. J Biol Chem. 1962 Mar;237:853–858. [PubMed] [Google Scholar]
- Gross R., Coles N. W. Adenosine triphosphatase in isolated membranes of Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1322–1326. doi: 10.1128/jb.95.4.1322-1326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkenscheid J. C., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XIX. Occurrence and properties of a (Na+-K+)-activated ATPase in Escherichia coli. Biochim Biophys Acta. 1968 Jan 8;151(1):204–211. doi: 10.1016/0005-2744(68)90175-7. [DOI] [PubMed] [Google Scholar]
- MARSH C., MILITZER W. Thermal enzymes. VII. Further data on an adenosinetriphosphatase. Arch Biochem Biophys. 1956 Feb;60(2):433–438. doi: 10.1016/0003-9861(56)90448-9. [DOI] [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- SOLOMON A. K. Ion transport in single cell populations. Biophys J. 1962 Mar;2(2 Pt 2):79–95. doi: 10.1016/s0006-3495(62)86949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Azzi A. Cation binding to submitochondrial particles. Biochim Biophys Acta. 1968 Apr 29;150(3):473–481. doi: 10.1016/0005-2736(68)90147-8. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Azzone G. F. Ion transport in liver mitochondria. VI. The role of surface binding on aerobic Ca++translocation. J Biol Chem. 1968 Oct 10;243(19):5132–5138. [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., GREENWALT J. W., LOW H. The hydrolysis of adenosine triphosphate by cell fractions of Bacillus megaterium. I. Localization and general characteristics of the enzymic activities. J Biol Chem. 1962 Mar;237:847–852. [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P. The sensitivity of a kidney ATPase to ouabain and to sodium and potassium. Biochim Biophys Acta. 1961 Aug 19;51:622–624. doi: 10.1016/0006-3002(61)90633-3. [DOI] [PubMed] [Google Scholar]
- Yayashi M., Uchida R. A cation activated adenosinetriphosphatase in cell membranes of halophilic Vibrio parahaemolyticus. Biochim Biophys Acta. 1965 Oct 25;110(1):207–209. doi: 10.1016/s0926-6593(65)80113-8. [DOI] [PubMed] [Google Scholar]


