Abstract
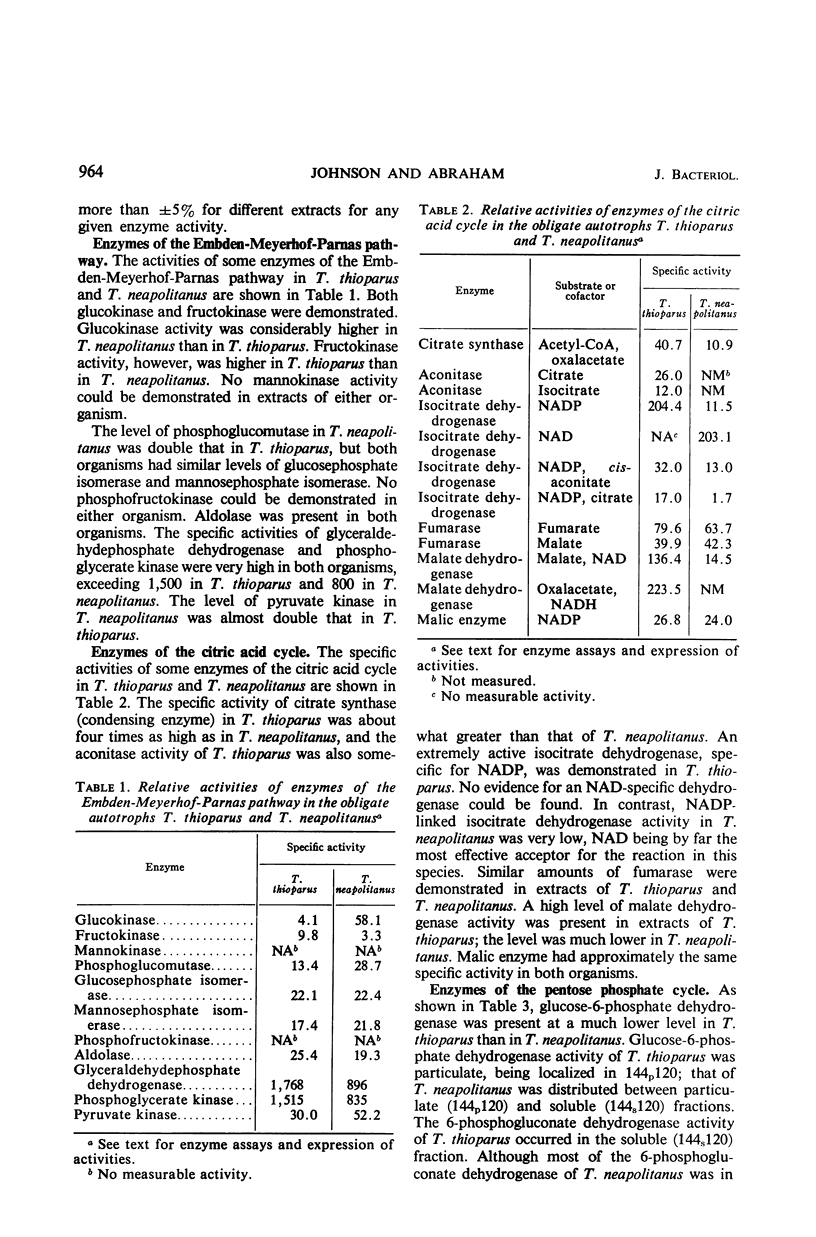
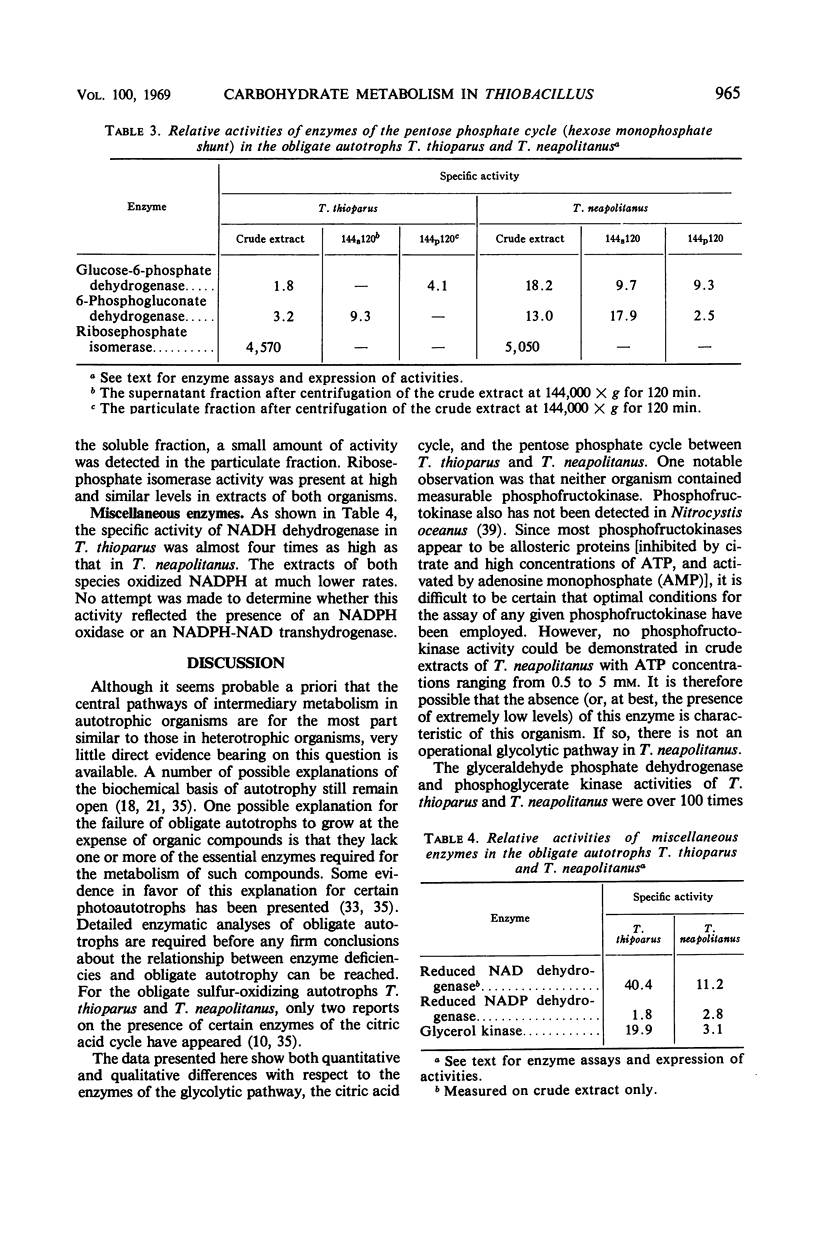
Levels of enzymes operative in the Embden-Meyerhof-Parnas (glycolytic) pathway, pentose phosphate cycle, citric acid cycle, and certain other phases of intermediary carbohydrate metabolism have been compared in Thiobacillus thioparus and T. neapolitanus. All enzymes of the glycolytic pathway except phosphofructokinase were demonstrated in both organisms. There were some striking quantitative differences between the two organisms with respect to the activities of the individual enzymes of the glycolytic pathway and the citric acid cycle. Qualitative differences were also found: the isocitrate dehydrogenase activity of T. thioparus is strictly nicotinamide adenine dinucleotide phosphate (NADP)-dependent, whereas that of T. neapolitanus is primarily nicotinamide adenine dinucleotide-dependent, activity with NADP being low; the glucose-6-phosphate dehydrogenase of T. thioparus is particulate, whereas that of T. neapolitanus is partly soluble and partly particulate; the 6-phosphogluconate dehydrogenase of T. thioparus is soluble, that of T. neapolitanus is partly soluble and partly particulate. All enzymes which function in the carbon reduction cycle were present at very high levels. In contrast, enzymes which operate exclusively in cycles other than the carbon reduction cycle were present at low levels. Of the enzymes not operative in the carbon reduction cycle that were examined, isocitric dehydrogenase had the highest specific activity. Both organisms possessed reduced nicotinamide adenine dinucleotide dehydrogenase activity. The qualitative and quantitative aspects of the data are discussed in relation to possible biochemical explanations of obligate autotrophy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., BORREBAEK B., CHAIKOFF I. L. EFFECT OF DIETARY CARBOHYDRATE AND GLUCOKINASE AND MANNOKINASE ACTIVITIES OF VARIOUS RAT TISSUES. J Nutr. 1964 Jul;83:273–288. doi: 10.1093/jn/83.3.273. [DOI] [PubMed] [Google Scholar]
- ABRAHAM S., FITCH W. M., CHAIKOFF I. L. Mannose metabolism and the demonstration of mannokinase and phosphomannoisomerase activities in the lactating rat mammary gland. Arch Biochem Biophys. 1961 May;93:278–282. doi: 10.1016/0003-9861(61)90262-4. [DOI] [PubMed] [Google Scholar]
- ABRAHAM S., KOPELOVICH L., KERKOF P. R., CHAIKOFF I. L. METABOLIC CHARACTERISTICS OF PREPARATIONS OF ISOLATED SHEEP THYROID GLAND CELLS. I. ACTIVITY LEVELS OF ENZYMES CONCERNED WITH GLYCOLYSIS AND THE TRICARBOXYLIC ACID CYCLE. Endocrinology. 1965 Feb;76:178–190. doi: 10.1210/endo-76-2-178. [DOI] [PubMed] [Google Scholar]
- BLUMENTHAL M. D., ABRAHAM S., CHAIKOFF I. L. DIETARY CONTROL OF LIVER GLUCOKINASE ACTIVITY IN THE NORMAL RAT. Arch Biochem Biophys. 1964 Feb;104:215–224. doi: 10.1016/s0003-9861(64)80006-0. [DOI] [PubMed] [Google Scholar]
- BORREBAEK B., ABRAHAM S., CHAIKOFF I. L. ENZYMIC REMOVAL OF GLUCOSE 6-PHOSPHATE FROM FRUCTOSE 6-PHOSPHATE PREPARATIONS. Anal Biochem. 1964 Jul;8:367–372. doi: 10.1016/0003-2697(64)90071-5. [DOI] [PubMed] [Google Scholar]
- COOPER R. C. EVIDENCE FOR THE PRESENCE OF CERTAIN TRICARBOXYLIC ACID CYCLE ENZYMES IN THIOBACILLUS THIOPARUS. J Bacteriol. 1964 Sep;88:624–629. doi: 10.1128/jb.88.3.624-629.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. L., Beck J. V. Evidence for the Calvin cycle and hexose monophosphate pathway in Thiobacillus ferrooxidans. J Bacteriol. 1967 Oct;94(4):1052–1059. doi: 10.1128/jb.94.4.1052-1059.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Vishniac W. Oxidative phosphorylation in extracts of thiobacillus X. Biochem Z. 1965 Aug 6;342(3):272–287. [PubMed] [Google Scholar]
- Ida S., Alexander M. Permeability of Nitrobacter agilis to Organic Compounds. J Bacteriol. 1965 Jul;90(1):151–156. doi: 10.1128/jb.90.1.151-156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. F., Moriarty D. J., Nicholas D. J. Deoxyribonucleic acid base composition and taxonomy of thiobacilli and some nitrifying bacteria. J Gen Microbiol. 1968 Aug;53(1):53–60. doi: 10.1099/00221287-53-1-53. [DOI] [PubMed] [Google Scholar]
- Johnson E. J., Abraham S. Assimilation and metabolism of exogenous organic compounds by the strict autotrophs Thiobacillus thioparus and Thiobacillus neapolitanus. J Bacteriol. 1969 Mar;97(3):1198–1208. doi: 10.1128/jb.97.3.1198-1208.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Problems of the autotrophic micro-organisms. Sci Prog. 1967 Spring;55(217):35–51. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayeux J. V., Johnson E. J. Effect of adenosine monophosphate, adenosine diphosphate, and reduced nicotinamide adenine dinucleotide on adenosine triphosphate-dependent carbon dioxide fixation in the autotroph Thiobacillus neapolitanus. J Bacteriol. 1967 Aug;94(2):409–414. doi: 10.1128/jb.94.2.409-414.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Shafia F., Wilkinson R. F., Jr Growth of Ferrobacillus ferrooxidans on organic matter. J Bacteriol. 1969 Jan;97(1):256–260. doi: 10.1128/jb.97.1.256-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A., Kelly D. P. Reduced nicotinamide adenine dinucleotide oxidation by Thiobacillus neapolitanus and Thiobacillus strain C. J Bacteriol. 1968 May;95(5):1962–1963. doi: 10.1128/jb.95.5.1962-1963.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. J., Watson S. W. Autotrophy in Nitrosocystis oceanus. J Bacteriol. 1968 Nov;96(5):1640–1648. doi: 10.1128/jb.96.5.1640-1648.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


