Abstract
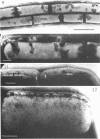
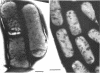
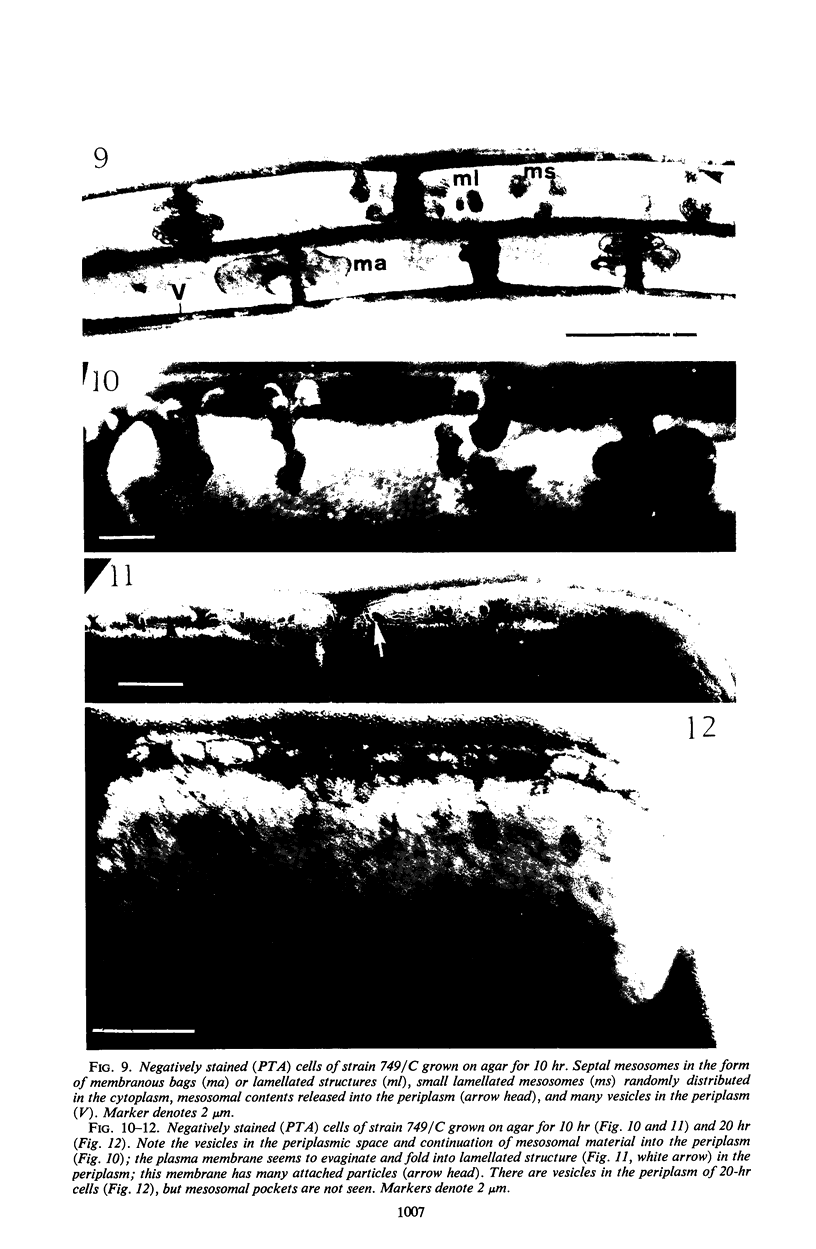
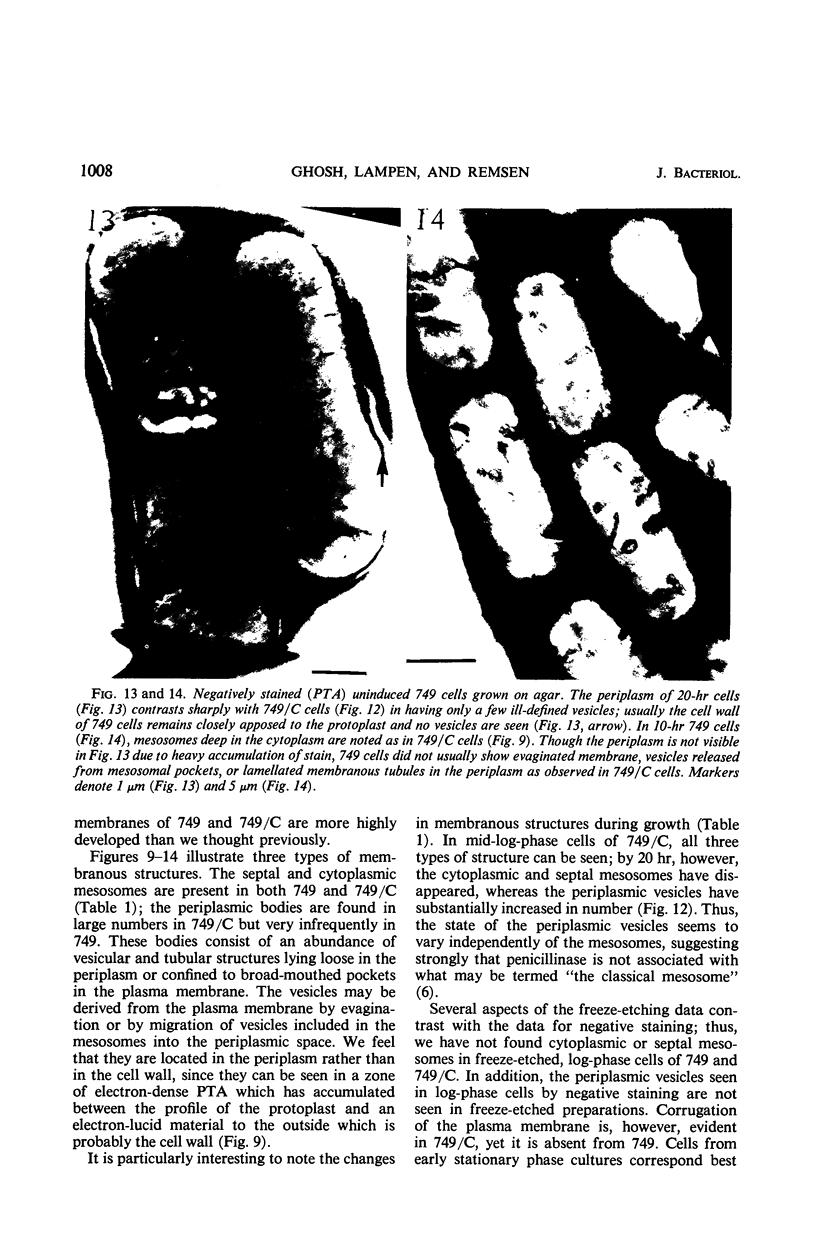
Bacillus licheniformis strain 749/C (constitutive for penicillinase formation) and uninduced cells of strain 749 (penicillinase-inducible) were examined after freezeetching. In the early stationary phase, strain 749/C organisms had clusters of vesicles (30 to 40 nm in diameter) on the outer surface of the plasma membrane. These are randomly distributed on the membrane, including the region of septum formation. The vesicles are not intimately associated with the plasma membrane, and their inner and outer surfaces are devoid of particles. Periplasmic vesicles were not detected by freeze-etching in strain 749 (uninduced) or in young cells of 749/C; however, the membrane of mid-logarithmic phase 749/C cells had a corrugated appearance. Negatively stained 749/C cells (logarithmic phase) also showed many vesicular and tubular bodies in the periplasm as well as septal and cytoplasmic mesosomes of typical morphology. The periplasmic structures appear to be formed either by evagination of plasma membrane or by migration of vesicular bodies from the membranous pockets of the cytoplasm. Stationary phase cells of 749/C still have many periplasmic vesicular bodies; however, the mesosomes are greatly reduced both in number and size. In sharp contrast, strain 749 organisms have very few structures similar to the periplasmic bodies of strain 749/C. These findings support our previous view that penicillinase-producing cells of 749/C have periplasmic membranous structures that are rare in the uninduced strain 749, though there is some lack of correspondence between freeze-etching, negative staining, and thin section data. These structures may be important for the retention or storage of penicillinase in the cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. Cell-bound penicillinase of Bacillus licheniformis; properties and purification. J Gen Microbiol. 1967 Aug;48(2):249–259. doi: 10.1099/00221287-48-2-249. [DOI] [PubMed] [Google Scholar]
- Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann N Y Acad Sci. 1965 Oct 13;125(2):658–676. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- Nanninga N. Structural features of mesosomes (chondrioids) of Bacillu subtilis after freeze-etching. J Cell Biol. 1968 Nov;39(2):251–263. doi: 10.1083/jcb.39.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Remsen C. C. The fine structure of frozen-etched Bacillus cereus spores. Arch Mikrobiol. 1966 Sep 8;54(3):266–275. doi: 10.1007/BF00408999. [DOI] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Characteristics of penicillinase secretion by growing cells and protoplasts of Bacillus licheniformis. J Bacteriol. 1969 Feb;97(2):820–826. doi: 10.1128/jb.97.2.820-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. PLASMOLYSIS IN BACILLUS MEGATERIUM. J Bacteriol. 1965 Apr;89:1151–1154. doi: 10.1128/jb.89.4.1151-1154.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]