Abstract
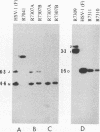
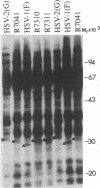
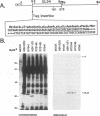
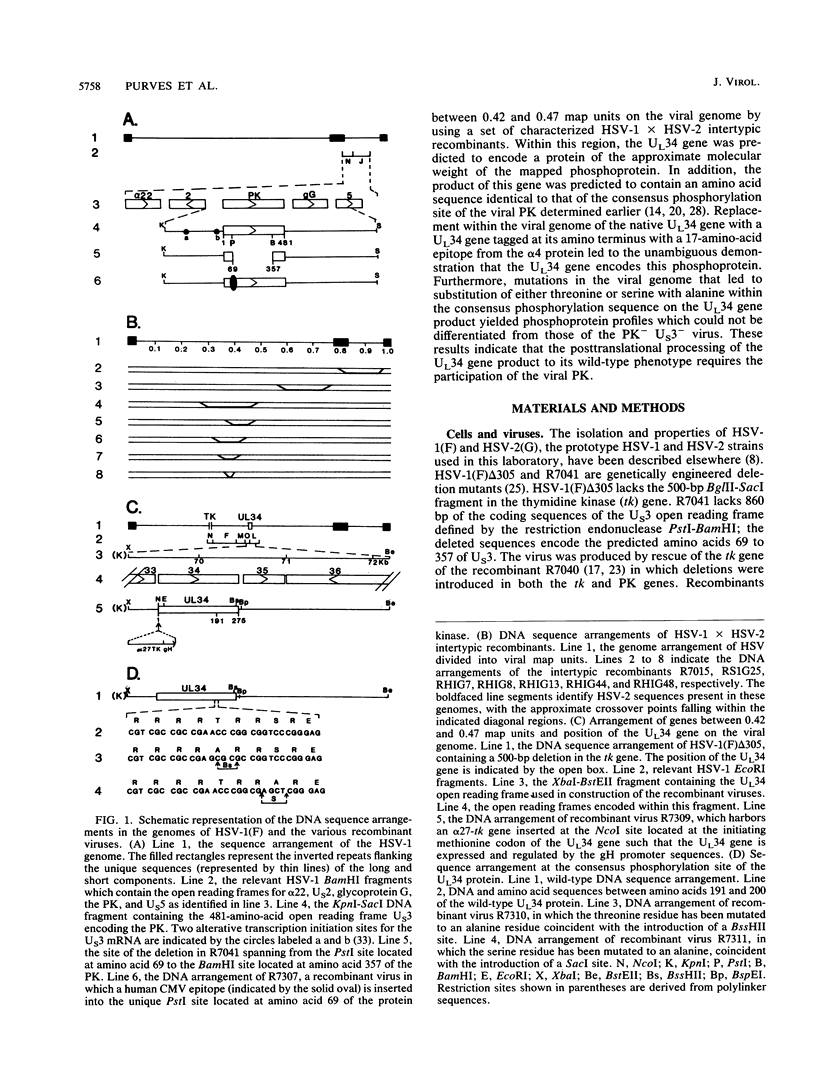
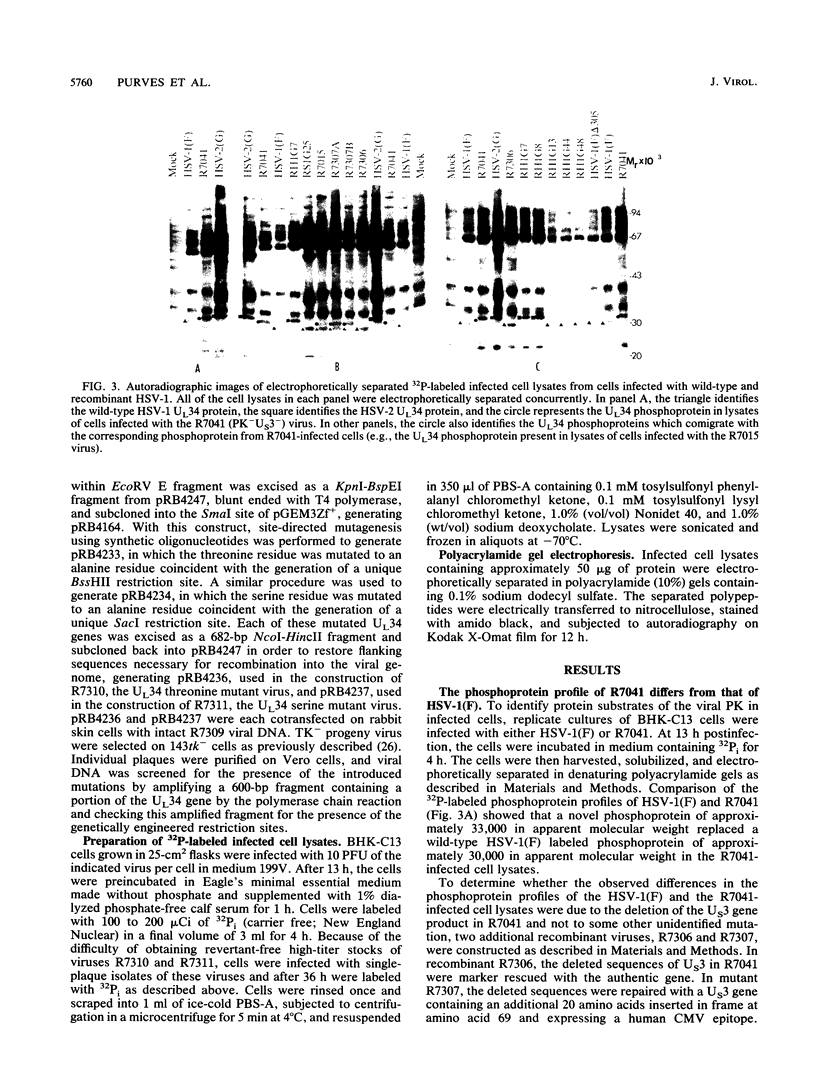
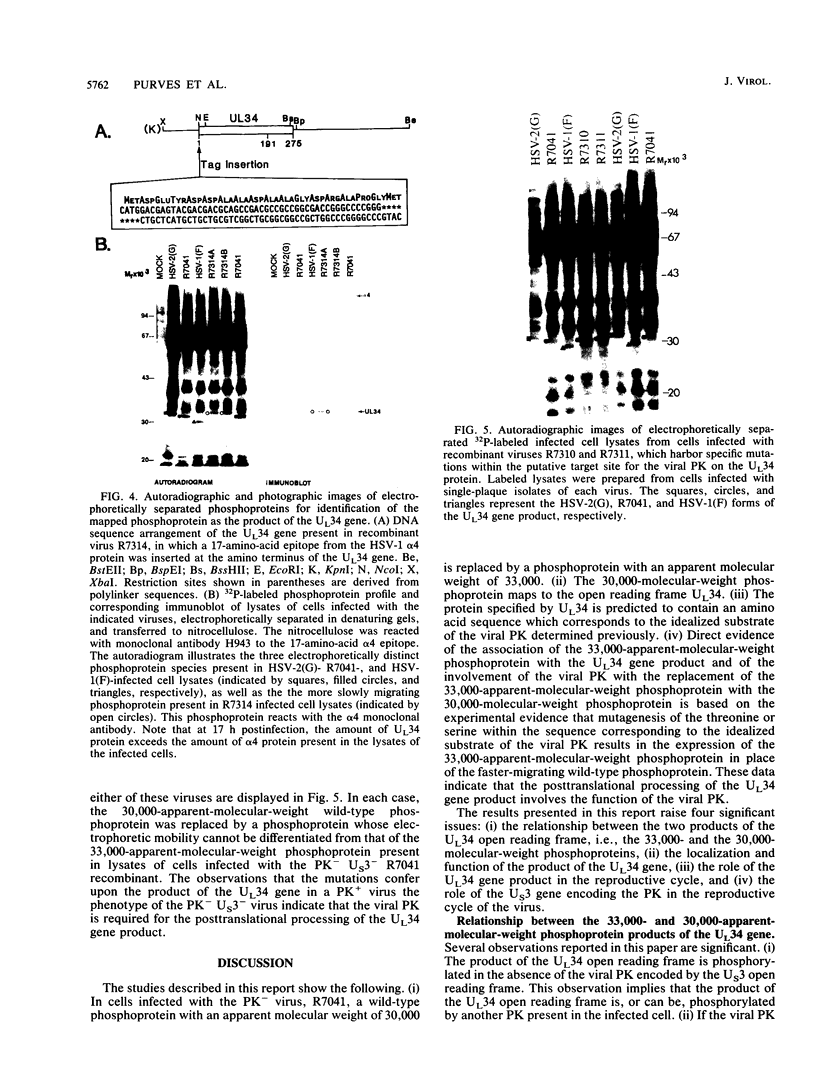
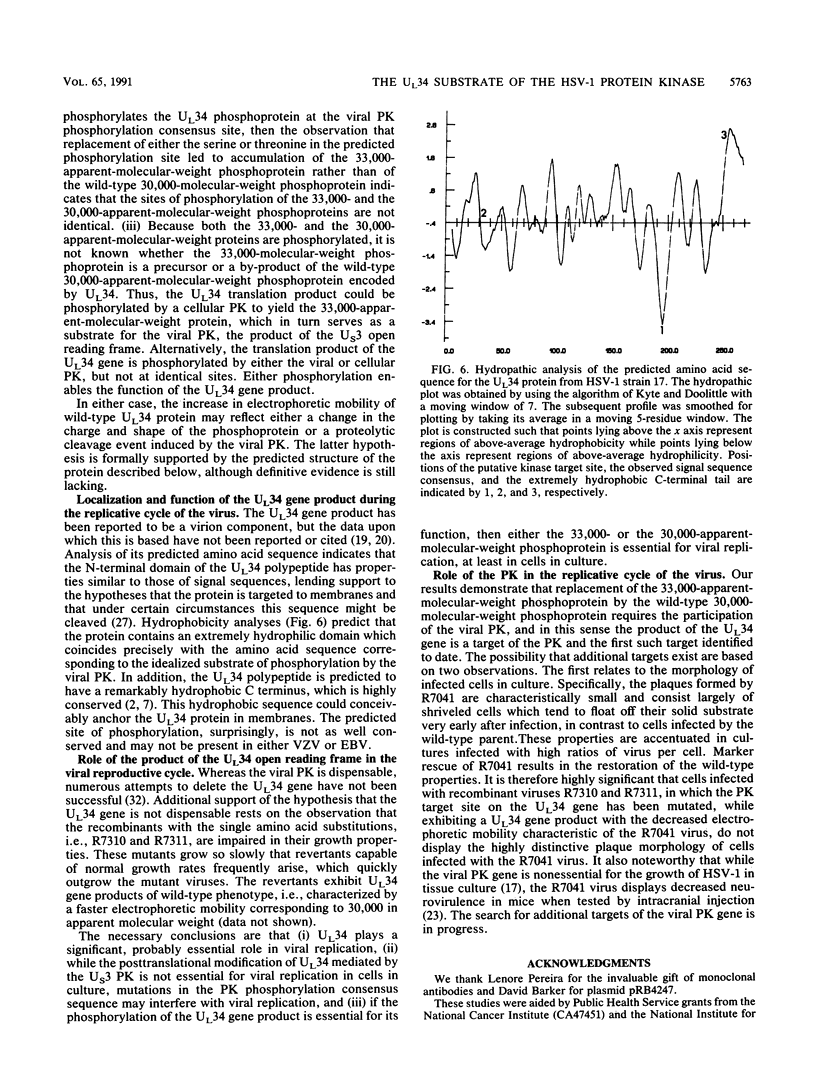
Earlier studies have shown that a herpes simplex virus 1 (HSV-1) open reading frame, US3, encodes a novel protein kinase and have characterized the cognate amino acid sequence which is phosphorylated by this enzyme. This report identifies an apparently essential viral phosphoprotein whose posttranslational processing involves the viral protein kinase. Analyses of viral proteins phosphorylated in the course of productive infection revealed a phosphoprotein whose mobility was viral protein kinase and serotype dependent. Thus, the corresponding HSV-1 and HSV-2 phosphoproteins differ in their electrophoretic mobilities, and the phosphoprotein specified by the HSV-1 mutant deleted in US3 (R7041) differs from that of the corresponding HSV-1 and HSV-2 proteins. Analyses of HSV-1 x HSV-2 recombinants mapped the phosphoprotein between 0.42 and 0.47 map units on the prototype HSV-1 DNA map. Within this region, the UL34 open reading frame was predicted to encode a protein of appropriate molecular weight which would also contain the consensus target site for phosphorylation by the viral protein kinase as previously defined with synthetic peptides. Replacement of the native UL34 gene with a UL34 gene tagged with a 17-amino-acid epitope from the alpha 4 protein identified this gene as encoding the phosphoprotein. Finally, mutagenesis of the predicted phosphorylation site on UL34 in the viral genome, and specifically the substitution of threonine or serine with alanine in the product of the UL34 gene, yielded phosphoproteins whose electrophoretic mobilities could not be differentiated from that of the US3- mutant. We conclude that the posttranslational processing of the UL34 gene product to its wild-type phenotype requires the participation of the viral protein kinase. While the viral protein kinase is not essential for viral replication in cells in culture, the UL34 gene product itself may not be dispensable.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Frame M. C., Purves F. C., McGeoch D. J., Marsden H. S., Leader D. P. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J Gen Virol. 1987 Oct;68(Pt 10):2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hubenthal-Voss J., Houghten R. A., Pereira L., Roizman B. Mapping of functional and antigenic domains of the alpha 4 protein of herpes simplex virus 1. J Virol. 1988 Feb;62(2):454–462. doi: 10.1128/jvi.62.2.454-462.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M., Stevely W. S., Leader D. P. Partial purification and characterization of a new phosphoprotein kinase from cells infected with pseudorabies virus. Eur J Biochem. 1985 Oct 1;152(1):57–65. doi: 10.1111/j.1432-1033.1985.tb09163.x. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Deana A. D., Marchiori F., Purves F. C., Pinna L. A. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim Biophys Acta. 1991 Feb 19;1091(3):426–431. doi: 10.1016/0167-4889(91)90210-o. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Purves F. C. The herpesvirus protein kinase: a new departure in protein phosphorylation? Trends Biochem Sci. 1988 Jul;13(7):244–246. doi: 10.1016/0968-0004(88)90157-0. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991 Jan;65(1):206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Maguire H. F., Hoeffler J. P., Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991 May 10;252(5007):842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986 Feb 25;14(4):1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Mavromara-Nazos P., Sears A. E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988 Jan;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Deana A. D., Marchiori F., Leader D. P., Pinna L. A. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim Biophys Acta. 1986 Nov 28;889(2):208–215. doi: 10.1016/0167-4889(86)90106-0. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Katan M., Leader D. P. Complete purification of the pseudorabies virus protein kinase. Eur J Biochem. 1987 Sep 15;167(3):507–512. doi: 10.1111/j.1432-1033.1987.tb13366.x. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Katan M., Stevely W. S., Leader D. P. Characteristics of the induction of a new protein kinase in cells infected with herpesviruses. J Gen Virol. 1986 Jun;67(Pt 6):1049–1057. doi: 10.1099/0022-1317-67-6-1049. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Longnecker R. M., Leader D. P., Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987 Sep;61(9):2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., McGeoch D. J. Detailed analysis of the mRNAs mapping in the short unique region of herpes simplex virus type 1. Nucleic Acids Res. 1985 Feb 11;13(3):953–973. doi: 10.1093/nar/13.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Zhou Z. Y., Judd A., Cartwright C. A., Robinson W. S. The hepatitis B virus-encoded transcriptional trans-activator hbx appears to be a novel protein serine/threonine kinase. Cell. 1990 Nov 16;63(4):687–695. doi: 10.1016/0092-8674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Zhang G., Stevens R., Leader D. P. The protein kinase encoded in the short unique region of pseudorabies virus: description of the gene and identification of its product in virions and in infected cells. J Gen Virol. 1990 Aug;71(Pt 8):1757–1765. doi: 10.1099/0022-1317-71-8-1757. [DOI] [PubMed] [Google Scholar]