Abstract
Infection of cattle with the protozoan Theileria parva results in uncontrolled T lymphocyte proliferation resulting in lesions resembling multicentric lymphoma. Parasitized cells exhibit autocrine growth characterized by persistent translocation of the transcriptional regulatory factor nuclear factor κB (NFκB) to the nucleus and consequent enhanced expression of interleukin 2 and the interleukin 2 receptor. How T. parva induces persistent NFκB activation, required for T cell activation and proliferation, is unknown. We hypothesized that the parasite induces degradation of the IκB molecules which normally sequester NFκB in the cytoplasm and that continuous degradation requires viable parasites. Using T. parva-infected T cells, we showed that the parasite mediates continuous phosphorylation and proteolysis of IκBα. However, IκBα reaccumulated to high levels in parasitized cells, which indicated that T. parva did not alter the normal NFκB-mediated positive feedback loop which restores cytoplasmic IκBα. In contrast, T. parva mediated continuous degradation of IκBβ resulting in persistently low cytoplasmic IκBβ levels. Normal IκBβ levels were only restored following T. parva killing, indicating that viable parasites are required for IκBβ degradation. Treatment of T. parva-infected cells with pyrrolidine dithiocarbamate, a metal chelator, blocked both IκB degradation and consequent enhanced expression of NFκB dependent genes. However treatment using the antioxidant N-acetylcysteine had no effect on either IκB levels or NFκB activation, indicating that the parasite subverts the normal IκB regulatory pathway downstream of the requirement for reactive oxygen intermediates. Identification of the critical points regulated by T. parva may provide new approaches for disease control as well as increase our understanding of normal T cell function.
Pathogen-mediated lymphoproliferation involving nuclear factor κB (NFκB) activation is the defining feature of several diseases, most notably human T-lymphotropic virus, type I, induced adult T-cell leukemia (1–3), progression of bovine leukemia virus infection to B cell lymphoma (4, 5), and East Coast Fever caused by Theileria parva (6). T. parva is a protozoal pathogen of cattle that replicates in the cytoplasm of lymphocytes (7), inducing lymphoblastoid transformation resulting in clonal expansion of the infected cell (8, 9). These transformed cells, primarily T cells, disseminate to lymphoid and nonlymphoid tissues, including the lung, kidney, and, intestine, where they mimic the behavior of lymphoid tumors (10, 11). Remarkably unlike true lymphomas, T. parva-induced transformation can be reversed by killing the parasite (9, 10–12). This drug-induced reversal to normal lymphocyte regulation indicates that T. parva alone is sufficient and responsible for transformation. Our goal is to identify the mechanism used by T. parva to subvert normal signaling pathways with the expectation that this knowledge may provide new approaches for disease control as well as increasing our understanding of normal T cell function.
T. parva-infected lymphocytes are capable of continuous growth without addition of exogenous growth factors (13, 14). This autocrine growth is, at least partially, attributable to parasite-induced expression of both interleukin 2 (IL-2) and the α-chain of the IL-2 receptor (IL-2Rα) (9, 13–16). Treatment with the theilericidal drug BW720c (a protozoal-specific hydronaphthoquinone) results in down-regulation of IL-2 and IL-2Rα expression and restoration of growth factor dependence (9, 12). Both IL-2 and IL-2Rα are transcriptionally regulated by cis-acting κB enhancer elements that are, in turn, controlled by dimeric complexes of the NFκB family of transcription factors (17, 18). Normally sequestered in the cytoplasm, NFκB regulatory proteins, principally the p50/Rel A heterodimer, are transiently translocated to the T cell nucleus following activation by antigen plus CD28 costimulation, mitogens, or cytokines, such as tumor necrosis factor-α (18, 19). Similarly, T. parva infection of T cells specifically induces nuclear translocation of NFκB and binding to the κB enhancer (6). Importantly however, NFκB translocation is persistent in the parasitized cell and requires viable T. parva (6). This indicates that the normal regulation of NFκB is specifically altered by the protozoan.
In T cells and in developing hematopoeitic organs, IκBα is the major inhibitor of the p50/RelA complex and sequesters the heterodimer in the cytoplasm via preferential binding to RelA and masking of the nuclear localization signals (18, 20–22). In response to antigen or cytokine activation, IκBα is phosphorylated and targeted for degradation in the proteosome (23–25). The freed p50/Rel A complex is translocated to the nucleus where it binds the κB element resulting in transcriptional activation of genes required for proliferation, including IL-2 and IL-2Rα (18, 19, 26). Importantly, IκBα is also transcriptionally regulated by a κB enhancer and therefore subsequent increased IκBα synthesis leads to re-established sequestration of p50/RelA in the cytoplasm (18, 27, 28). This autoregulatory loop ensures that the NFκB-induced gene expression is normally transient and tightly regulated. Persistent NFκB activation is also associated with, and appears to require, degradation of a second inhibitor, IκBβ (29, 30). Unlike IκBα, IκBβ gene expression is not controlled by κB elements and restoration of normal cytoplasmic IκBβ levels for NFκB retention is therefore not directly linked to prior degradation (29).
How does T. parva infection induce the persistent NFκB activation required for the IL-2 autocrine loop and lymphoproliferation? We hypothesize that the parasite induces degradation of the IκB inhibitory molecules allowing NFκB translocation and that continuous degradation is dependent on the parasite. Consequently, this mechanism should be fully reversible by killing the parasite and restoring normal control of lymphocyte growth. In this paper, we report the testing of this hypothesis.
MATERIALS AND METHODS
Cell Lines and Cultures.
T. parva-parasitized cloned bovine cell lines were derived and cultivated as previously described (9, 31). The principal cell line used was α/β T cell receptor positive (TCR+), CD4+, and CD8−. Three additional cell lines were used to show the generality of the findings, these were a second α/β TCR+/CD4+/CD8− cell line, an α/β TCR+/CD4−/CD8+ cell line, and a γ/δ TCR+ cell line. To eliminate the parasite from infected cells, BW720c, a hydronaphthoquinone derivative, was added daily to a final concentration of 50 ng/ml. The theilericidal properties of BW720c, which does not affect mammalian cells even at much higher concentrations, have been described in detail (12). Chemical inhibitors or activators were added to the final concentrations indicated: cycloheximide (50 μg/ml), Con A (5 μg/ml), N-acetylcysteine (NAc, 25 μg/ml), okadaic acid (40 μM), and pyrrolidine dithiocarbamate (PDTC, 25 μg/ml). Whole cell, nuclear, and cytoplasmic lysates were prepared as previously described (32).
Immunoblot Analysis.
PAGE and immunoblot detection of NFκB and IκB proteins were done as described (33). Rabbit anti-peptide polyclonal antibodies (Santa Cruz Biotechnology) with the following specificities were used: IκBα (C-terminal 21 amino acids); IκBβ (C-terminal 20 amino acids); NFκB1/p50 (C-terminal 20 amino acids); RelA/p65 (C-terminal 20 amino acids); and as a negative control, Grb-2 (C-terminal 20 amino acids). The molecular sizes of the IκB and NFκB proteins are highly conserved among species: IκBα, ≈40 kDa; IκBβ, 49 kDa; NFκB1/p50, 50 kDa; and RelA/p65, 65 kDa.
Coimmunoprecipitation of IκB-NFκB Complexes.
Cytoplasmic extracts were incubated sequentially with primary rabbit anti-peptide antibodies and protein A-agarose, each for 1 hr at 4°C. Agarose beads were washed 4x by centrifugation and resuspension. Complexes were eluted from the agarose beads using Laemmli sample buffer (containing 2% SDS and 1.5% DTT) and collected in the supernatant following centrifugation. Detection of IκB/NFκB components in the supernatant was done as referenced above.
Cell Transfection and Chloramphnicol Acetyltransferase (CAT) Assay.
Plasmids −121/+232 HIV-CAT and −76/+232 HIV-CAT were derived by modification of pBLCAT5 containing the bacterial CAT gene and have been described (6). In plasmid −121/+232 HIV-CAT (abbreviated HIV-CAT), the CAT gene is under control of an HIV-1 enhancer with two κB elements and the promoter region of the enhancer. Plasmid −76/+232 HIV-CAT (ΔHIV-CAT) is identical except for deletion of the region containing the κB elements (6). Transfection was done using electroporation and, prior to treatment with PDTC or NAc, cells were pooled to eliminate variation in CAT activity due to differences in transfection efficiency among aliquots. CAT activity was determined as described (34).
RESULTS
T. parva Infection of T Cells Regulates IκBα and IκBβ.
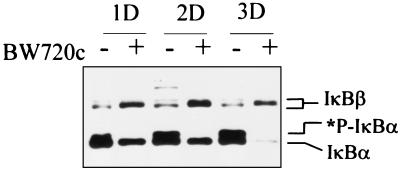
Immunoblot analysis of cell lysates from 1 × 107 CD4+ T cells using a combination of specific anti-IκBα and anti-IκBβ antibodies revealed higher levels of IκBα and lower levels of IκBβ in parasite infected cells as compared with the same cloned cell line cleared of the parasite (Fig. 1). Immunoblots of lysates from parasite infected cells or BW720c treated cells normalized to equal protein quantity (data not shown) were similar to Fig. 1, which used lysates from equal numbers of cells.
Figure 1.
T. parva infection induces increased levels of IκBα and phosphorylated IκBα and decreased total IκBβ levels. T. parva-infected T cells were left untreated (−) or treated daily with BW720C to clear the parasite (+). Lysates were prepared from 107 cells collected following 1, 2, or 3 days in culture and IκBα and IκBβ levels determined by immunoblot analysis. *P-IκBα designates phosphorylated IκBα.
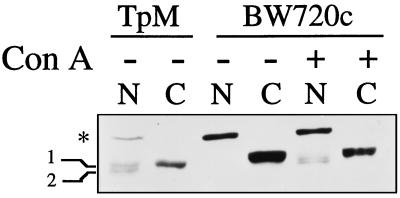
The immunoblots of IκBβ in whole cell lysates revealed multiple molecular size forms (Fig. 1). In cells cleared of the parasite the large molecular size form was exclusively nuclear (∗ in Fig. 2) and the smaller forms exclusively cytoplasmic (designated 1 and 2 in Fig. 2). All forms were diminished in T. parva infected cells with the smaller molecular size forms found in both nuclear and cytoplasmic extracts (Fig. 2). Nuclear translocation of the smaller IκBβ forms also occurred following Con A re-stimulation of cells cleared of the parasite. (Fig. 2).
Figure 2.
T. parva infection induces nuclear expression of IκBβ normally restricted to the cytoplasm. Nuclear and cytoplasmic extracts were prepared from T. parva infected cells (TpM) or the same cloned cell line cleared of the parasite (BW720C), either unstimulated (−) or stimulated with Con A (+). Nuclear and cytoplasmic distribution of the IκBβ molecular size forms was determined by immunoblot analysis using an anti-IκBβ specific antibody. The position of the constitutive nuclear form of IκBβ (∗) and the constitutive cytoplasmic phosphorylated (labeled 1), and unphosphorylated (labeled 2) forms are designated in the left margin.
IκBα and IκBβ Bind p65 in the Cytoplasm of T. parva-Parasitized T Cells.
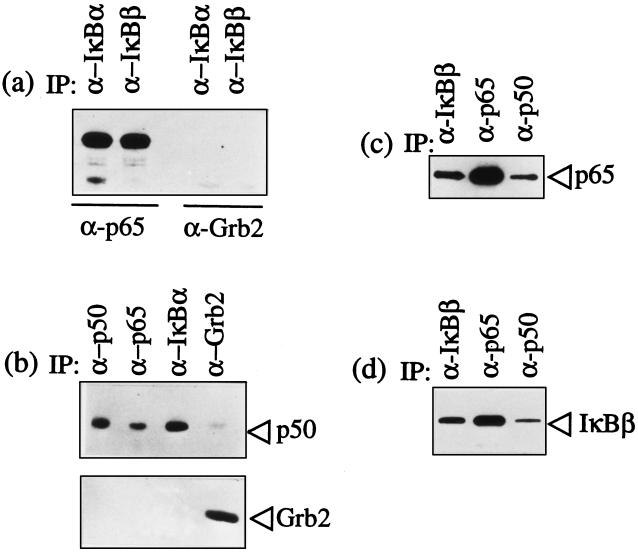
The nuclear translocation of NFκB in the presence of high levels of cytoplasmic IκBα indicated that the parasite alters regulation of the normal cytoplasmic retention of p65 RelA by IκBα. To determine whether the parasite directly inhibited binding of p65 RelA to IκBα or IκBβ, cytoplasmic lysates of parasite infected cells were immunoprecipitated with specific antibodies for either IκBα or IκBβ. Immunoprecipitates were then analyzed in immunoblots using an anti-p65 antibody or, as a negative control, an anti-Grb2 antibody. Both IκBα and IκBβ bound cytoplasmic p65 RelA (Fig. 3a). Next, to test whether the complete p65 RelA/p50 heterodimer was bound by the IκB proteins, coimmunoprecipitations using specific antibodies for p50, p65, IκBα, or IκBβ were done. Immunoblot analysis of p50, p65, and IκBα immunoprecipitates with antibodies against p50 revealed that p50 associates with both p65 RelA and IκBα within the cytoplasm of infected cells (Fig. 3b). Similarly, immunoblot analysis of p50, p65 RelA, and IκBβ immunoprecipitates with anti-p65 (Fig. 3c) and anti-IκBβ (Fig. 3d) antibodies demonstrated binding of the p65 and p50 in the expected heterodimer and concomitant binding with IκBβ. Therefore the increased IκBα in the cytoplasm of parasitized cells is still capable of binding p65 RelA/p50 heterodimers, as is the less abundant cytoplasmic IκBβ.
Figure 3.
T. parva infection does not abrogate IκBα and IκBβ binding to p65 RelA and p50/p65 RelA heterodimers. The specific antibodies used in immunoprecipitation are designated above each lane in the figures. The immunoprecipitated complexes were then analyzed using immunoblots to identify coprecipitated components. (a) IκBα and IκBβ bind p65 RelA. Immunoblot analysis of IκBα and IκBβ immunoprecipitates with anti-p65 RelA antibodies detected bound p65 RelA. The immunoblot with antibody against Grb2 was used as a negative control for nonspecific complex formation. (b) p50 is bound to both IκBα and p65 RelA. Immunoblot analysis of p65 and IκBα immunoprecipitates with anti-p50 antibodies detected bound p50. The p50 and Grb2 immunoprecipitates were included as positive and negative controls, respectively. (c) p65 RelA is bound to both IκBβ and p50. Immunoblot analysis of p50 and IκBβ immunoprecipitates with anti-p65 antibodies detected bound p65. The p65 immunoprecipitate was included as a positive control. (d) IκBβ is bound to both p50 and p65 RelA. Immunoblot analysis of p50 and p65 RelA immunoprecipitates with anti-IκBβ antibodies detected bound IκBβ. The IκBβ immunoprecipitate was included as a positive control.
IκBα and IκBβ Are Constitutively Degraded in T. parva-Parasitized T Cells.
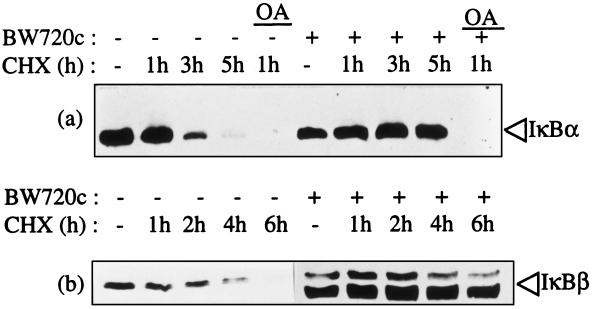
The presence of both high levels of IκBα and phosphorylated IκBα in parasitized cells suggested that continuous phosphorylation and signaling for degradation may be occurring but at a slower rate than new IκBα synthesis. To test this, cells were incubated with cycloheximide to inhibit new cellular protein synthesis. Lysates of equal cell numbers were then immunoblotted using specific antibodies for IκBα (Fig. 4a) or IκBβ (Fig. 4b). The degradation of IκBα was evident in parasite infected cells incubated with cycloheximide for 3 hr and by 5 hr, IκBα was barely detectable (Fig. 4a). Similarly, IκBβ degradation was evident in parasite infected cells in which new synthesis had been inhibited for 4 hr, with no observable IκBβ following 6 hr of inhibition (Fig. 4b). In contrast, the IκBα and IκBβ levels in the same cloned cell line cleared of the parasite were relatively stable in absence of new translation (Fig. 4 a and b). Thus the parasite induces continuous degradation of both IκBα and IκBβ.
Figure 4.
T. parva infection mediates continuous degradation of IκBα and IκBβ. T. parva infected T cells (−) or the same cloned cell line cleared of the parasite using BW720C (+) were treated with cycloheximide (CHX) to inhibit protein synthesis. The duration of CHX treatment in hr (h) prior to cell harvest is designated at the top of each figure. Okadaic acid (OA), which blocks the serine/threonine phosphatase inhibitors types PP1 and PP2A, was included as a positive control for IκBα degradation. IκBα (a) and IκBβ levels (b) were determined by immunoblot analysis.
Parasite-Induced IκB Degradation Is Inhibited by PDTC but Not by NAc.
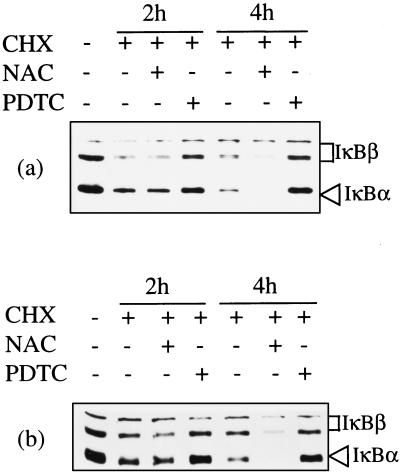
Induction of NFκB translocation in T cells by cytokines or CD28-mediated costimulation is regulated, in part, by generation of reactive oxygen intermediates that initiate IκBα degradation (35–39). The ability to partially block proliferation of T. parva infected cells by using a nonspecific lipooxygenase inhibitor suggested that reactive oxygen intermediates may also play a critical role in parasite-mediated regulation of NFκB (54). To determine whether T. parva continuous NFκB translocation was dependent on generation of reactive oxygen intermediates, equal numbers of parasite infected T cells were either left untreated or treated with the antioxidant NAc for 5 hr and then incubated with cycloheximide prior to harvest to allow detection of continuous IκBα and IκBβ degradation. PDTC, a metal chelator that has been shown to consistently block NFκB translocation in primary and transformed T cell lines, was also tested. Diminished IκBα and IκBβ levels were evident after 2 hr of cycloheximide incubation (Fig. 5a). Treatment with PDTC-inhibited parasite-induced degradation of both IκBα and IκBβ (Fig. 5a). In contrast, NAc treatment apparently enhanced the T. parva-mediated degradation of both IκBα and IκBβ (as compared with untreated cells at 4 hr, Fig. 5a). These contrasting effects of PDTC and NAc on IκB degradation were also observed when each lane was loaded with equal protein quantity (Fig. 5b), rather than equal cell numbers (Fig. 5a).
Figure 5.
T. parva-mediated continuous degradation of IκBα and IκBβ is inhibitable by PDTC but not by the antioxidant NAc. T. parva-infected cells were left untreated or treated with PDTC or NAc for 5 hr and then incubated with cycloheximide (CHX). IκB levels were determined by immunoblot analysis.
Parasite-Induced NFκB Activation Is Inhibited by PDTC but Not by NAc.
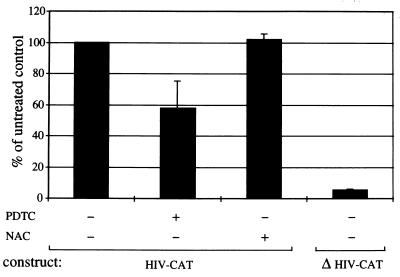
To test whether the effects of PDTC and NAc on IκB degradation altered NFκB translocation, an electrophoretic mobility shift assay was used to detect NFκB binding to 32P-labeled oligonucleotides representing consensus κB recognition sites (6). While neither PDTC or NAc completely prevented NFκB translocation and DNA binding in T. parva infected cells, PDTC treatment diminished NFκB binding (data not shown). To determine if this PDTC effect resulted in significant inhibition of NFκB directed reporter gene activity, parasitized CD4+ T cells were transfected with the plasmid HIV-CAT, in which CAT expression is κB dependent (6). As shown in Fig. 6, there was a significant reduction in CAT activity in T. parva parasitized cells treated with PDTC as compared with untreated or NAc treated parasitized cells. The NFκB dependence of the CAT activity was confirmed by the loss of CAT activity in parasitized cells transfected with the mutant plasmid ΔHIV-CAT, in which both κB motifs had been deleted (6) (Fig. 6).
Figure 6.
T. parva-mediated NFκB dependent gene expression is inhibitable by PDTC but not NAc. Results are expressed as the percentage of CAT activity in untreated HIV-CAT transfected cells. Results include SDs.
DISCUSSION
Nuclear translocation of NFκB is required for T cell proliferation and therefore its tight regulation is critical for control of lymphoproliferation. Phosphorylation and degradation of cytoplasmic IκBα is the first step to release p50/RelA heterodimers for translocation (17–19). As shown in the present study, cytoplasmic IκBα is continuously degraded in T. parva infected cells and this enhanced proteolysis is dependent on viable parasites. However, the rapid induction of new synthesis results in an overall increased IκBα level in parasitized cells, indicating that κB-mediated up-regulation of IκBα expression occurs normally (27, 28) and is not subverted by T. parva infection. Nonetheless, the high level of reaccumulated IκBα is not able to re-establish control over NFκB translocation. These results are consistent with the biphasic activation model of persistent NFκB activation in which a first phase of IκBα proteolysis must be accompanied by IκBβ degradation (19, 29, 30, 40). Critically, IκBβ is also degraded in parasitized T cells but new synthesis is inadequate to restore normal levels, and therefore total IκBβ is decreased. Degradation of IκBβ also requires the continuous presence of viable parasites, with restoration of normal levels within 24 hr of T. parva killing. This restoration of normal IκBβ levels following clearance of the parasite occurs concomitantly with termination of NFκB translocation (6), κB dependent gene expression (6, 16, 41), and cellular proliferation (9). This is consistent with our hypothesis that active parasite-mediated IκBβ degradation is a key step in continuous lymphoproliferation.
IκBβ newly synthesized following stimulation of cells with IL-1 or lipopolysaccharide is unphosphorylated and allows persistent NFκB activation despite high levels of reaccumulated IκBα (29, 40). Unphosphorylated IκBβ binds p65/RelA, preventing association with reaccumulated IκBα, but without masking the p65/RelA nuclear localization signal (40). Consequently, the IκBβ-NFκB complex translocates to the nucleus and binds DNA κB enhancer sites (40). The enhancer bound complex is protected against IκBα-mediated re-export to the cytoplasm and provides prolonged expression of κB regulated genes (29, 40). The continuous degradation of IκBβ in T. parva infected T cells fits this model of persistent activation by requiring continual new synthesis of the unphosphorylated IκBβ. Consistent with this model, the small unphosphorylated IκBβ form, previously identified in the nucleus of cells stimulated with IL-1 or lipopolysaccharide (40), is present in the nucleus of T. parva infected cells and disappears following BW720c treatment. This nuclear expression of unphosphorylated IκBβ in parasitized T cells correlates with the persistent NFκB translocation and enhanced κB-dependent gene expression (6, 16, 41).
T. parva-mediated lymphoproliferation mimics the clonal expansion of antigen-stimulated T cells that requires IκB degradation, NFκB translocation, and enhanced IL-2 and IL-2Rα expression. The critical difference is that T. parva subverts the normal antigen signaling pathways to cause unlimited proliferation. T. parva infection bypasses the normal requirement for TCR activation as shown by the lack of TCRζ and CD3ɛ phosphorylation in parasitized cells (42). Similarly, inhibition of early TCR-dependent signaling pathways using ascomycin or cyclosporin A to target calcineurin, or bisindolylmaleimide to block protein kinase C has no effect on T. parva-mediated proliferation (42). In contrast, c-Jun N-terminal kinases are constitutively activated in infected cells and activation is dependent on the parasite (42). Importantly, activation of c-Jun N-terminal kinases in stimulated T cells has recently been shown to be coordinately regulated with IκBα degradation (via an IκBα kinase in a 700 kDa complex) due to the common upstream mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase-1 (MEKK-1) (43). Consequently, T. parva activation of MEKK-1, either directly or via the small GTPases such as Rac1, Cdc42, and Ras, could result in both the observed c-Jun N-terminal kinase kinases activation (42) and the continuous IκB degradation reported in this study.
Alternatively, T. parva may directly target IκB for degradation using either a parasite-encoded kinase or phosphatase inhibitor. Phosphorylation of IκB molecules on N-terminal serine residues triggers subsequent ubiquination and proteolysis in the proteosome (23–25). This inducible phophorylation is primarily mediated in normal cells by one or more IκB kinases contained in large multiprotein complexes (44, 45). An active parasite kinase with similar specificity would explain the continuous phosphorylation and degradation observed in infected cells. In addition to the inducible IκB kinase complexes, cellular casein kinase II constitutively phosphorylates the C-terminal region of IκBα in normal cells to regulate IκBα stability and NFκB binding (46–48). Interestingly, the host cell casein kinase II is constitutively activated in T. parva-infected cells (49). Furthermore, the parasite encodes its own casein kinase II (49–51), thereby presenting an attractive possibility for direct parasite regulation of IκBα. In contrast to kinases, inhibitors of the serine/threonine phosphatases PP1 and PP2A also allow IκBα phosphorylation, degradation, and NFκB activation (18, 19, 45), a function that could be mimicked by a parasite encoded inhibitor.
The inhibition of IκB proteolysis in parasitized cells by the metal chelator PDTC but not by the antioxidant NAc suggests that the degradative pathway is not dependent on reactive oxygen intermediates. Oxygen intermediates appear to play a role early in the signaling pathway leading to IκBα breakdown (52). For example, Rac1 triggers an NFκB activation pathway upstream of MEKK-1 and is NAc inhibitable (53). If the oxygen intermediates are required only prior to MEKK-1 activation, then the insensitivity of T. parva to NAc would indicate that the parasite subverts normal signaling pathways at or downstream of MEKK-1. We will test this hypothesis using transfection of parasitized cells with dominant negative MEKK-1 mutants (43). The results of this experiment will direct future approaches to identifying the key points of parasite-induced IκB degradation.
The mechanism of persistent NFκB activation in the T. parva CD4+ T cell line, continuous degradation of IκBα and IκBβ, was also identified in an additional parasitized CD4+ T cell line and in T. parva-infected α/β TCR+/CD4−/CD8+ and γ/δ TCR+ cells (data not shown). Interestingly, this mechanism appears to be convergent between retroviruses (30, 40) and, as shown here, protozoa. Identification of the critical signaling pathways subverted by these diverse pathogens may provide new approaches for control of lymphoproliferative diseases as well as increasing our understanding of normal lymphocyte function.
Acknowledgments
We thank Terry McElwain, Wendy Brown, Dorothy French, and Barbara von Beust for review of the manuscript. This work was supported by the Swiss National Science Foundation (No. 31.43328.95), Novartis (CIBA–Geigy Jubiläums-Stiftung), the U.S. Department of Agriculture, and Washington State University.
ABBREVIATIONS
- MEKK-1
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase-1
- IL-2
interleukin 2
- IL-2Rα
IL-2 receptor α-chain
- PDTC
pyrrolidine dithiocarbamate
- NAc
N-acetylcysteine
- CAT
chloramphenicol acetyltransferase
- HIV-CAT
−121/+232 HIV-CAT
- TCR
T cell receptor
References
- 1.Ruben S, Poteat H, Tan T H, Kawakami K, Roeder R, Haseltine W, Rosen C A. Science. 1988;241:89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- 2.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 3.Crenon I, Beraud C, Simard P, Montagne J, Veschambre P, Jalinot P. Oncogene. 1993;8:867–875. [PubMed] [Google Scholar]
- 4.Mauxion F, Jamieson C, Yoshida M, Arai K, Sen R. Proc Natl Acad Sci USA. 1991;88:2141–2145. doi: 10.1073/pnas.88.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks P A, Nyborg J K, Cockerell G L. J Virol. 1995;69:6005–6009. doi: 10.1128/jvi.69.10.6005-6009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov V, Stein B, Baumann I, Dobbelaere D A, Herrlich P, Williams R O. Mol Cell Biol. 1989;9:4677–4686. doi: 10.1128/mcb.9.11.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvin A D, Ocama J G, Spooner P R. Res Vet Sci. 1982;33:298–304. [PubMed] [Google Scholar]
- 8.Brown C G, Stagg D A, Purnell R E, Kanhai G K, Payne R C. Nature (London) 1973;245:101–103. doi: 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- 9.Dobbelaere D A, Coquerelle T M, Roditi I J, Eichhorn M, Williams R O. Proc Natl Acad Sci USA. 1988;85:4730–4734. doi: 10.1073/pnas.85.13.4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison W I, Buscher G, Murray M, Emery D L, Masake R A, Cook R H, Wells P W. Exp Parasitol. 1981;52:248–260. doi: 10.1016/0014-4894(81)90080-1. [DOI] [PubMed] [Google Scholar]
- 11.Irvin A D, Stagg D A, Kanhai G K, Brown C G. Nature (London) 1975;253:549–550. doi: 10.1038/253549a0. [DOI] [PubMed] [Google Scholar]
- 12.Hudson A T, Randall A W, Fry M, Ginger C D, Hill B, Latter V S, McHardy N, Williams R B. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 13.Brown W C, Shaw M K, Conrad P A, Dolan T T. Exp Parasitol. 1989;68:308–325. doi: 10.1016/0014-4894(89)90113-6. [DOI] [PubMed] [Google Scholar]
- 14.Brown W C, Logan K S. Parasite Immunol (Oxf) 1986;8:189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 15.Eichhorn M, Dobbelaere D. Res Immunol. 1995;146:89–99. doi: 10.1016/0923-2494(96)80242-2. [DOI] [PubMed] [Google Scholar]
- 16.Coquerelle T M, Eichhorn M, Magnuson N S, Reeves R, Williams R O, Dobbelaere D A. Eur J Immunol. 1989;19:655–659. doi: 10.1002/eji.1830190413. [DOI] [PubMed] [Google Scholar]
- 17.Siebenlist U, Franzoso G, Brown K. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 18.Baeuerle P A, Henkel T. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin A S J. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Beg A A, Ruben S M, Scheinman R I, Haskill S, Rosen C A, Baldwin A S J. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 21.Beg A A, Sha W C, Bronson R T, Baltimore D. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 22.Baeuerle P A, Baltimore D. Genes Dev. 1989;3:1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 23.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 26.Grimm S, Baeuerle P A. Biochem J. 1993;290:297–308. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H, Bach F H. EMBO J. 1993;12:2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Proc Natl Acad Sci USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J E, Phillips R J, Erdjument Bromage H, Tempst P, Ghosh S. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 30.Good L, Sun S C. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emery D L. Vet Parasitol. 1981;9:1–16. doi: 10.1016/0304-4017(81)90002-9. [DOI] [PubMed] [Google Scholar]
- 32.Stein B, Kraemer M, Rahmsdorf H J, Ponta H, Herrlich P. J Virol. 1989;63:4540–4544. doi: 10.1128/jvi.63.11.4540-4544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronstein I, Voyta J C, Murphy O J, Bresnick L, Kricka L J. BioTechniques. 1992;12:748–753. [PubMed] [Google Scholar]
- 34.Seed B, Sheen J Y. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- 35.Los M, Schenk H, Hexel K, Baeuerle P A, Droge W, Schulze Osthoff K. EMBO J. 1995;14:3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer M, Schreck R, Baeuerle P A. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staal F J, Roederer M, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreck R, Rieber P, Baeuerle P A. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenk H, Klein M, Erdbrugger W, Droge W, Schulze Osthoff K. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suyang H, Phillips R, Douglas I, Ghosh S. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heussler V T, Eichhorn M, Reeves R, Magnuson N S, Williams R O, Dobbelaere D A. J Immunol. 1992;149:562–567. [PubMed] [Google Scholar]
- 42.Galley Y, Hagens G, Glaser I, Davis W C, Eichhorn M, Dobbelaere D A E. Proc Natl Acad Sci USA. 1997;94:5119–5124. doi: 10.1073/pnas.94.10.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee F S, Hagler J, Chen Z J, Maniatis T. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z J, Parent L, Maniatis T. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 45.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 46.Barroga C F, Stevenson J K, Schwarz E M, Verma I M. Proc Natl Acad Sci USA. 1995;92:7637–7641. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez M S, Michalopoulos I, Arenzana Seisdedos F, Hay R T. Mol Cell Biol. 1995;15:2413–2419. doi: 10.1128/mcb.15.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ernst M K, Dunn L L, Rice N R. Mol Cell Biol. 1995;15:872–882. doi: 10.1128/mcb.15.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ole MoiYoi O K, Brown W C, Iams K P, Nayar A, Tsukamoto T, Macklin M D. EMBO J. 1993;12:1621–1631. doi: 10.1002/j.1460-2075.1993.tb05807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ole MoiYoi O K, Sugimoto C, Conrad P A, Macklin M D. Biochemistry. 1992;31:6193–6202. doi: 10.1021/bi00142a004. [DOI] [PubMed] [Google Scholar]
- 51.ole MoiYoi O K. Science. 1995;267:834–836. doi: 10.1126/science.7846527. [DOI] [PubMed] [Google Scholar]
- 52.Schreck R, Baeuerle P A. Methods Enzymol. 1994;234:151–163. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- 53.Sulciner D J, Irani K, Yu Z X, Ferrans V J, Goldschmidt Clermont P, Finkel T. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaussepied M, Langsley G. Res Immunol. 1996;147:127–138. doi: 10.1016/0923-2494(96)83165-8. [DOI] [PubMed] [Google Scholar]