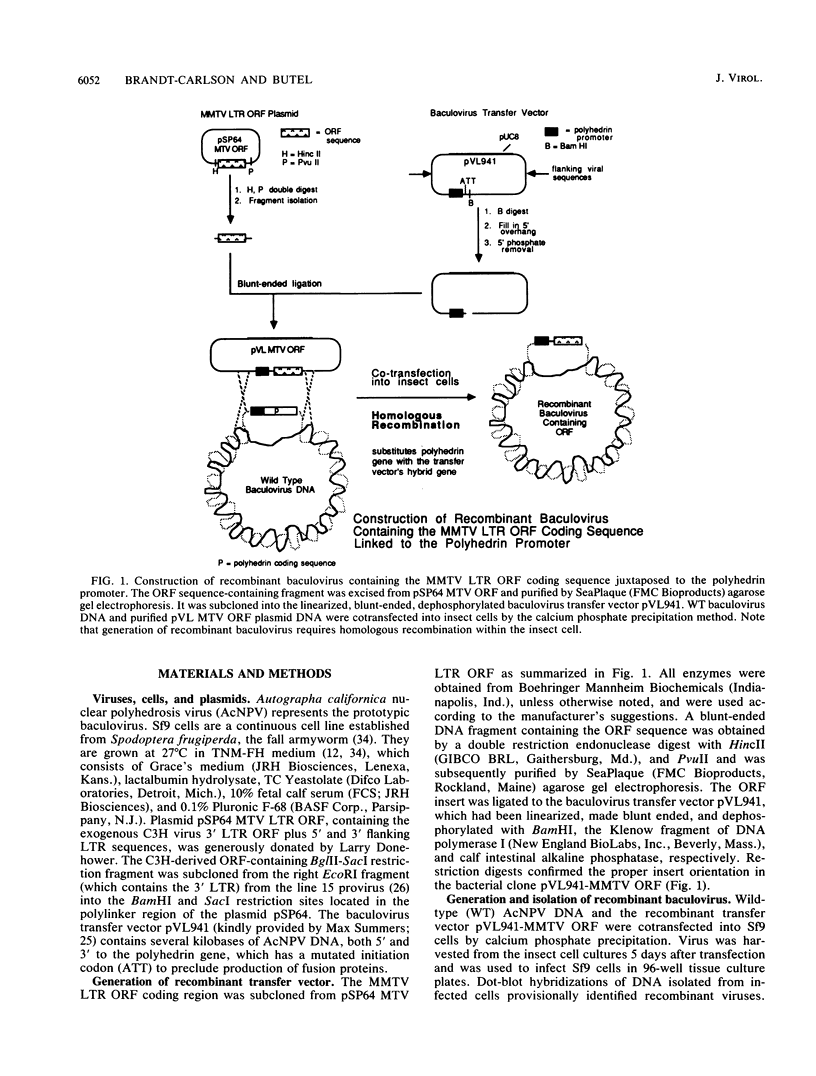
Abstract
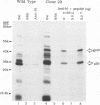
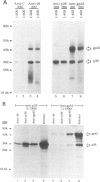
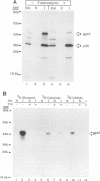
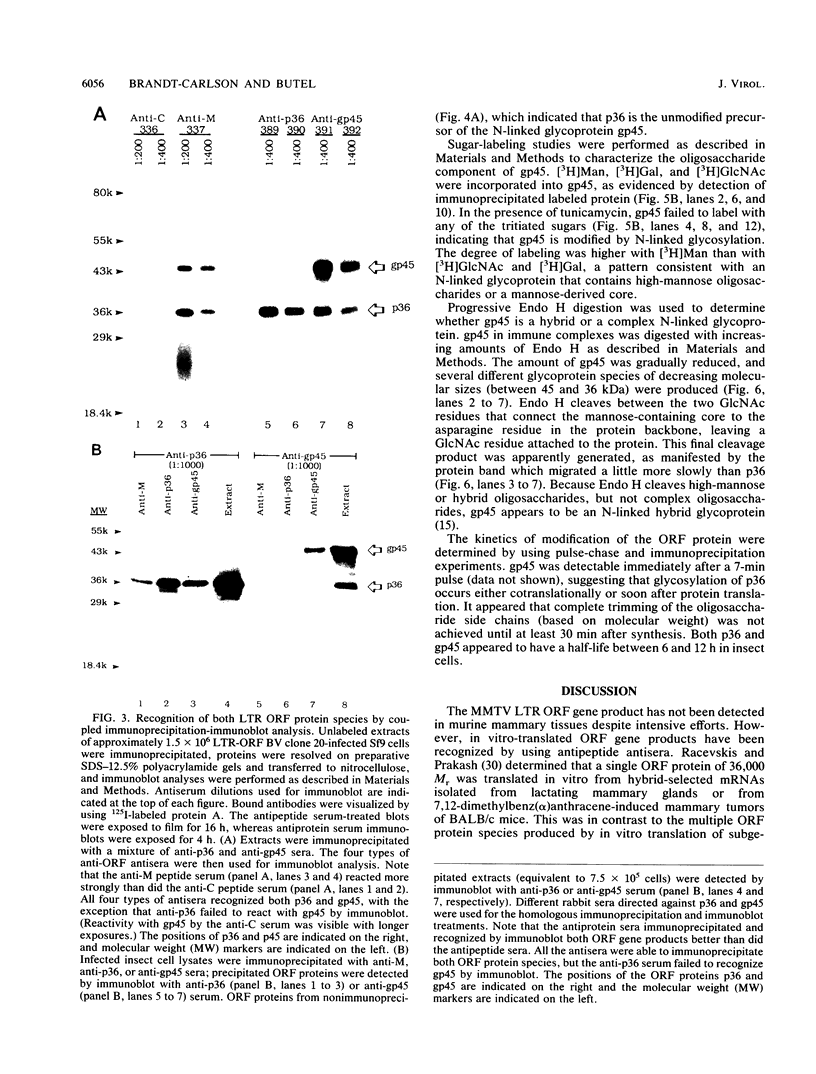
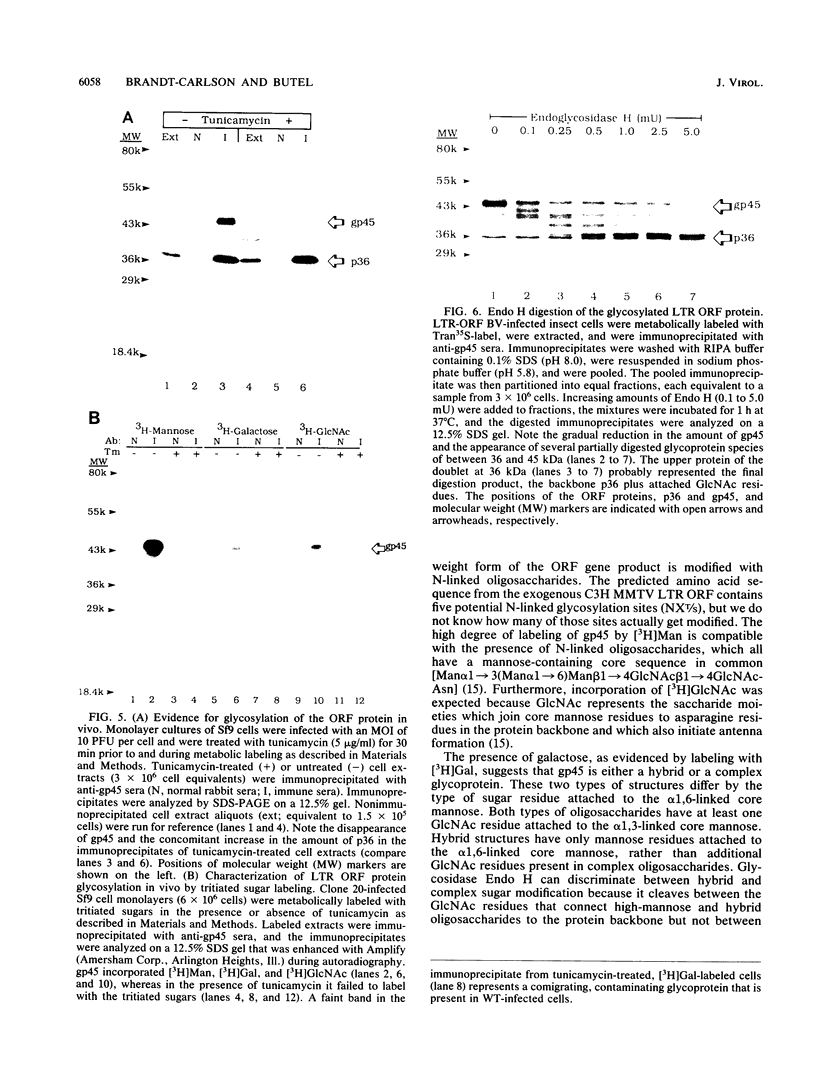
Mouse mammary tumor virus (MMTV) is a retrovirus that causes mammary tumors in susceptible mice. MMTV contains a unique open reading frame (ORF) in the unique 3' region of the proviral long terminal repeat (LTR) with the potential to encode a 36-kDa protein. However, the ORF gene product has not been detected in murine mammary tissues or cell lines. We utilized the baculovirus expression vector system to generate large amounts of the ORF protein. Putative ORF gene products of 36 and 45 kDa were detected as unique proteins in extracts of insect cells infected with recombinant baculovirus (LTR-ORF BV), and the identities of these proteins as viral gene products were confirmed immunologically. Antipeptide antisera were generated in rabbits against peptides chosen from computer-predicted hydrophilic regions of the ORF coding sequence. These antisera reacted specifically by immunoprecipitation and by immunoblot with the proteins expressed in LTR-ORF BV-infected insect cells, as well as with MMTV LTR ORF in vitro translation products. Polyclonal antisera were raised against two putative ORF protein species partially purified from insect cells. These sera specifically immunoprecipitated viral protein products translated in vitro. In vitro translation of MMTV LTR ORF transcripts in the presence of canine pancreatic microsomal membranes generated a higher-molecular-weight ORF gene product, indicating that the ORF protein is modified by N-linked glycosylation. This glycosylated ORF product comigrated with the larger ORF protein species produced in infected insect cells. The gp45 product was metabolically labeled with [3H] mannose, [3H] galactose, and [3H] N-acetyl-D-glucosamine in insect cells, whereas this incorporation was inhibited in the presence of tunicamycin. Digestion of gp45 with endoglycosidase H yielded the lower-molecular-weight ORF protein p36. These observations suggest that the ORF glycoprotein contains hybrid N-linked oligosaccharides. Demonstration of the modified nature of the ORF gene product will facilitate characterization of ORF protein expression in murine tissues.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Crouse C. A., Pauley R. J. Molecular cloning and sequencing of the MTV-1 LTR: evidence for a LTR sequence alteration. Virus Res. 1989 Feb;12(2):123–137. doi: 10.1016/0168-1702(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Peters G. In vitro synthesis of polypeptides encoded by the long terminal repeat region of mouse mammary tumour virus DNA. Nature. 1981 Jun 11;291(5815):511–513. doi: 10.1038/291511a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Jarvis D. L., Lanford R. E., Butel J. S. Structural comparisons of wild-type and nuclear transport-defective simian virus 40 large tumor antigens. Virology. 1984 Apr 15;134(1):168–176. doi: 10.1016/0042-6822(84)90282-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- King L. B., Lund F. E., White D. A., Sharma S., Corley R. B. Molecular events in B lymphocyte differentiation. Inducible expression of the endogenous mouse mammary tumor proviral gene, Mtv-9. J Immunol. 1990 Apr 15;144(8):3218–3227. [PubMed] [Google Scholar]
- Knepper J. E., Kittrell F. S., Medina D., Butel J. S. Spontaneous progression of hyperplastic outgrowths of the D1 lineage to mammary tumors: expression of mouse mammary tumor virus and cellular proto-oncogenes. Mol Carcinog. 1989;1(4):229–238. doi: 10.1002/mc.2940010405. [DOI] [PubMed] [Google Scholar]
- Knepper J. E., Medina D., Butel J. S. Activation of endogenous MMTV proviruses in murine mammary cancer induced by chemical carcinogen. Int J Cancer. 1987 Sep 15;40(3):414–422. doi: 10.1002/ijc.2910400322. [DOI] [PubMed] [Google Scholar]
- Knepper J. E., Medina D., Butel J. S. Differential expression of endogenous mouse mammary tumor virus genes during development of the BALB/c mammary gland. J Virol. 1986 Aug;59(2):518–521. doi: 10.1128/jvi.59.2.518-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988 Nov;167(1):56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Smith R., Brookes S., Dickson C. Conservation of protein coding potential in the long terminal repeats of exogenous and endogenous mouse mammary tumor viruses. J Virol. 1982 Jun;42(3):880–888. doi: 10.1128/jvi.42.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J. Expression of the protein product of the mouse mammary tumor virus long terminal repeat gene in phorbol ester-treated mouse T-cell-leukemia cells. J Virol. 1986 May;58(2):441–449. doi: 10.1128/jvi.58.2.441-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Prakash O. Proteins encoded by the long terminal repeat region of mouse mammary tumor virus: identification by hybrid-selected translation. J Virol. 1984 Sep;51(3):604–610. doi: 10.1128/jvi.51.3.604-610.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Erfle V., Brem G., Günzburg W. H. naf, a trans-regulating negative-acting factor encoded within the mouse mammary tumor virus open reading frame region. J Virol. 1990 Dec;64(12):6355–6359. doi: 10.1128/jvi.64.12.6355-6359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagle B. L., Lanford R. E., Medina D., Butel J. S. Expression of mammary tumor virus proteins in preneoplastic outgrowth lines and mammary tumors of BALB/cV mice. Cancer Res. 1984 May;44(5):2155–2162. [PubMed] [Google Scholar]
- Smith G. H., Young L. J., Benjamini F., Medina D., Cardiff R. D. Proteins antigenically related to peptides encoded by the mouse mammary tumour virus long terminal repeat sequence are associated with intracytoplasmic A particles. J Gen Virol. 1987 Feb;68(Pt 2):473–486. doi: 10.1099/0022-1317-68-2-473. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Butel J. S., Medina D., Cardiff R. D., Hager G. L. Transcription of mouse mammary tumor virus: identification of a candidate mRNA for the long terminal repeat gene product. J Virol. 1983 Apr;46(1):42–49. doi: 10.1128/jvi.46.1.42-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- van Klaveren P., Bentvelzen P. Transactivating potential of the 3' open reading frame of murine mammary tumor virus. J Virol. 1988 Nov;62(11):4410–4413. doi: 10.1128/jvi.62.11.4410-4413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]