Abstract
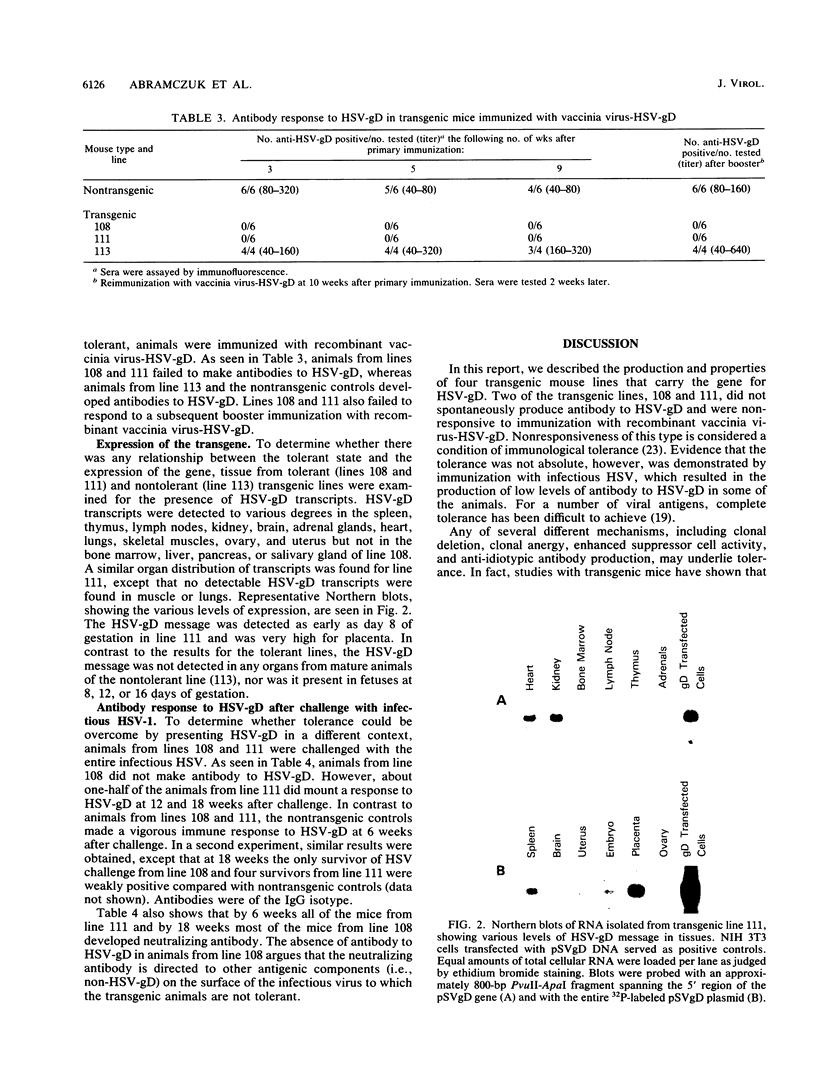
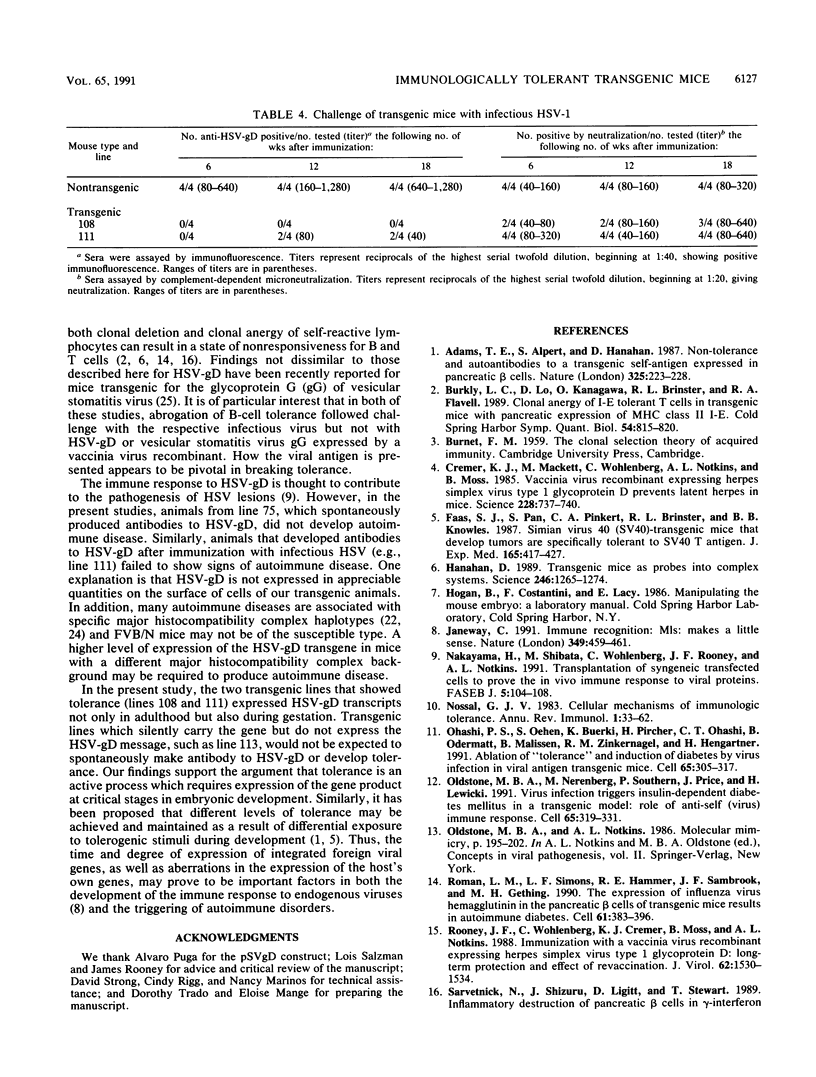
A construct containing the gene for glycoprotein D of herpes simplex virus (HSV-gD), under the control of the simian virus 40 early promoter, was microinjected into single-cell embryos, and four transgenic mouse lines were established. Three were homozygous (lines 75, 111, and 113) and one was hemizygous (line 108) for the HSV-gD gene. Examination of sera revealed that only one of the lines (line 75) spontaneously produced antibody to HSV-gD. Immunization of the other three lines with vaccinia virus-HSV-gD showed that one of them (line 113) responded by making antibody to HSV-gD, whereas the other two (lines 108 and 111) appeared to be immunologically tolerant. Evidence that tolerance was not absolute was obtained by immunization with infectious HSV, which resulted in an antibody response to HSV-gD in some of the animals from line 111. Examination of organs for HSV-gD mRNA revealed transcripts in the tolerant line (line 108) and in the partially tolerant line (line 111), but not in the nontolerant line (line 113), suggesting that the development of immunological tolerance requires active expression of the HSV-gD gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. E., Alpert S., Hanahan D. Non-tolerance and autoantibodies to a transgenic self antigen expressed in pancreatic beta cells. Nature. 1987 Jan 15;325(6101):223–228. doi: 10.1038/325223a0. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. Clonal anergy of I-E-tolerant T cells in transgenic mice with pancreatic expression of MHC class II I-E. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):815–820. doi: 10.1101/sqb.1989.054.01.095. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- Faas S. J., Pan S., Pinkert C. A., Brinster R. L., Knowles B. B. Simian virus 40 (SV40)-transgenic mice that develop tumors are specifically tolerant to SV40 T antigen. J Exp Med. 1987 Feb 1;165(2):417–427. doi: 10.1084/jem.165.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Transgenic mice as probes into complex systems. Science. 1989 Dec 8;246(4935):1265–1275. doi: 10.1126/science.2686032. [DOI] [PubMed] [Google Scholar]
- Janeway C. Immune recognition. Mls: makes a little sense. Nature. 1991 Feb 7;349(6309):459–461. doi: 10.1038/349459a0. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Shibata M., Wohlenberg C., Rooney J. F., Notkins A. L. Transplantation of syngeneic transfected cells to probe the in vivo immune response to viral proteins. FASEB J. 1991 Jan;5(1):104–108. doi: 10.1096/fasebj.5.1.1846831. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Roman L. M., Simons L. F., Hammer R. E., Sambrook J. F., Gething M. J. The expression of influenza virus hemagglutinin in the pancreatic beta cells of transgenic mice results in autoimmune diabetes. Cell. 1990 May 4;61(3):383–396. doi: 10.1016/0092-8674(90)90521-f. [DOI] [PubMed] [Google Scholar]
- Rooney J. F., Wohlenberg C., Cremer K. J., Moss B., Notkins A. L. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988 May;62(5):1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Shibata M., Puga A., Salata K. F., Bachurski C. J., Lerman M. I., Notkins A. L. Expression of a viral gene in insulin-producing cell lines renders them susceptible to immunological destruction. Diabetologia. 1989 Oct;32(10):709–715. doi: 10.1007/BF00274529. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., McDevitt H. O., Steinman L., Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989 Jun 2;57(5):709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Cooper S., Chambers J., Lazzarini R. A., Hengartner H., Arnheiter H. Virus-induced autoantibody response to a transgenic viral antigen. Nature. 1990 May 3;345(6270):68–71. doi: 10.1038/345068a0. [DOI] [PubMed] [Google Scholar]