Abstract
The mechanism of human immunodeficiency virus type 1 (HIV-1) cytopathicity is poorly understood and might involve formation of multinucleated giant cells (syncytia), single-cell lysis, or both. In order to determine the contributions of the fusion domain to syncytium formation, single-cell lysis, and viral infectivity and to clarify the molecular details of these events, insertion mutations were made in the portion of env encoding this sequence in the functional HIV-1 proviral clone HXB2. Viruses produced from these mutant clones were found to have a partial (F3) or complete (F6) loss of syncytium-forming ability in acutely infected CEM, Sup T1, and MT4 T-cell lines. During the early stage of acute infection by F6 virus, there was a loss of the syncytial cytopathic effect, which resulted in increased cell viability, and a 1.9- to 2.6-fold increase in virus yield in the cell lines tested. In the late stage of acute infection, the single-cell cytopathic effect of F6 virus was similar to that of the parental HXB2 virus. The F3 and F6 viruses were also found to have a 1.7- to 43-fold reduction in infectivity compared with the HXB2 virus. The mutant F3 and F6 and parental HXB2 envelope proteins were expressed in vaccinia virus, and the mutant envelope proteins were observed to be defective in their ability to form syncytia. BSC-40 cells infected with vaccinia virus recombinants revealed no differences in kinetics of cleavage, cell surface expression, or CD4 binding capacity of the mutant and parental envelope proteins. These results demonstrate that a loss of syncytium formation results in an attenuation of infectivity and a loss of the syncytial cytopathic effect without a loss of single-cell lysis. These mutants may reflect in tissue culture the changes observed in the HIV isolates in vivo during disease progression, which exhibit marked differences in syncytium production.
Full text
PDF
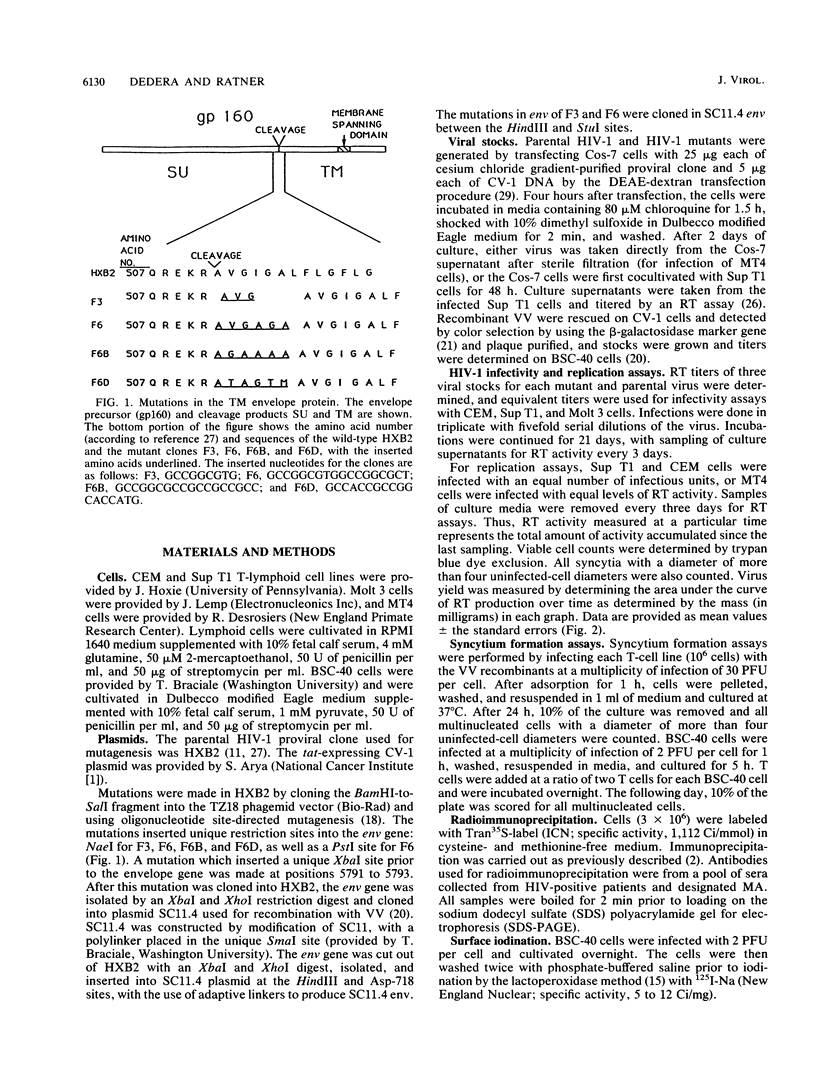






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Barin F., McLane M. F., Allan J. S., Lee T. H., Groopman J. E., Essex M. Virus envelope protein of HTLV-III represents major target antigen for antibodies in AIDS patients. Science. 1985 May 31;228(4703):1094–1096. doi: 10.1126/science.2986291. [DOI] [PubMed] [Google Scholar]
- Blattner W., Gallo R. C., Temin H. M. HIV causes AIDS. Science. 1988 Jul 29;241(4865):515–516. doi: 10.1126/science.3399881. [DOI] [PubMed] [Google Scholar]
- Bosch M. L., Earl P. L., Fargnoli K., Picciafuoco S., Giombini F., Wong-Staal F., Franchini G. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989 May 12;244(4905):694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- Brasseur R., Cornet B., Burny A., Vandenbranden M., Ruysschaert J. M. Mode of insertion into a lipid membrane of the N-terminal HIV gp41 peptide segment. AIDS Res Hum Retroviruses. 1988 Apr;4(2):83–90. doi: 10.1089/aid.1988.4.83. [DOI] [PubMed] [Google Scholar]
- Burny A., Bex F., Brasseur R., Khim M. C., Delchambre M., Horth M., Verdin E. Human immunodeficiency virus cell entry: new insights into the fusion mechanism. J Acquir Immune Defic Syndr. 1988;1(6):579–582. [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Tateno M., Levy J. A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988 Apr 1;240(4848):80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- Crane S. E., Clements J. E., Narayan O. Separate epitopes in the envelope of visna virus are responsible for fusion and neutralization: biological implications for anti-fusion antibodies in limiting virus replication. J Virol. 1988 Aug;62(8):2680–2685. doi: 10.1128/jvi.62.8.2680-2685.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedera D., Vander Heyden N., Ratner L. Attenuation of HIV-1 infectivity by an inhibitor of oligosaccharide processing. AIDS Res Hum Retroviruses. 1990 Jun;6(6):785–794. doi: 10.1089/aid.1990.6.785. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Doms R. W., Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1990 Jan;87(2):648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ratner L., Mitsuya H., Marselle L. M., Harper M. E., Broder S., Gallo R. C., Wong-Staal F. Infectious mutants of HTLV-III with changes in the 3' region and markedly reduced cytopathic effects. Science. 1986 Aug 8;233(4764):655–659. doi: 10.1126/science.3014663. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Bergeron L., Dorfman T., Haseltine W., Sodroski J. Attenuation of human immunodeficiency virus type 1 cytopathic effect by a mutation affecting the transmembrane envelope glycoprotein. J Virol. 1991 Jan;65(1):281–291. doi: 10.1128/jvi.65.1.281-291.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Cho E. S., Lemann W., Navia B. A., Price R. W. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986 Nov;45(6):635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J., Tilley S. A., Bona C., Zaghouani H., Gorny M. K., Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Rey M. A., Laurent A. G., McClure J., Krust B., Montagnier L., Hovanessian A. G. Transmembrane envelope glycoproteins of human immunodeficiency virus type 2 and simian immunodeficiency virus SIV-mac exist as homodimers. J Virol. 1990 Feb;64(2):922–926. doi: 10.1128/jvi.64.2.922-926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Fenyö E. M., Pavlakis G. N. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7200–7203. doi: 10.1073/pnas.86.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R., Cho E. S., Epstein L. G. Multinucleated giant cells and HTLV-III in AIDS encephalopathy. Hum Pathol. 1985 Aug;16(8):760–760. doi: 10.1016/s0046-8177(85)80245-8. [DOI] [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. Unexpectedly high levels of HIV-1 RNA and protein synthesis in a cytocidal infection. Science. 1988 Dec 16;242(4885):1554–1557. doi: 10.1126/science.3201245. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Meier C., Mann A. M., Chapman N., Wasiak A. Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS. Cell. 1988 May 6;53(3):483–496. doi: 10.1016/0092-8674(88)90168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersmette M., de Goede R. E., Al B. J., Winkel I. N., Gruters R. A., Cuypers H. T., Huisman H. G., Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988 Jun;62(6):2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Briesen H., Becker W. B., Henco K., Helm E. B., Gelderblom H. R., Brede H. D., Rübsamen-Waigmann H. Isolation frequency and growth properties of HIV-variants: multiple simultaneous variants in a patient demonstrated by molecular cloning. J Med Virol. 1987 Sep;23(1):51–66. doi: 10.1002/jmv.1890230107. [DOI] [PubMed] [Google Scholar]





