Abstract
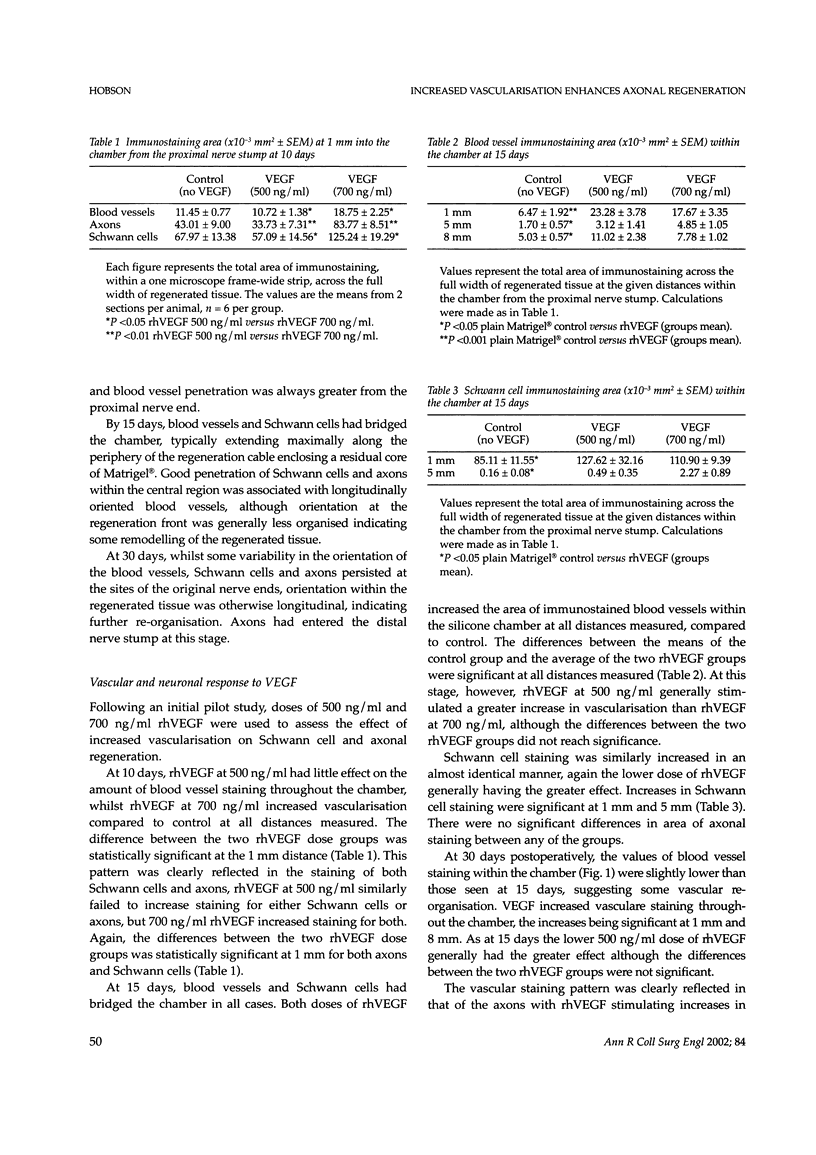
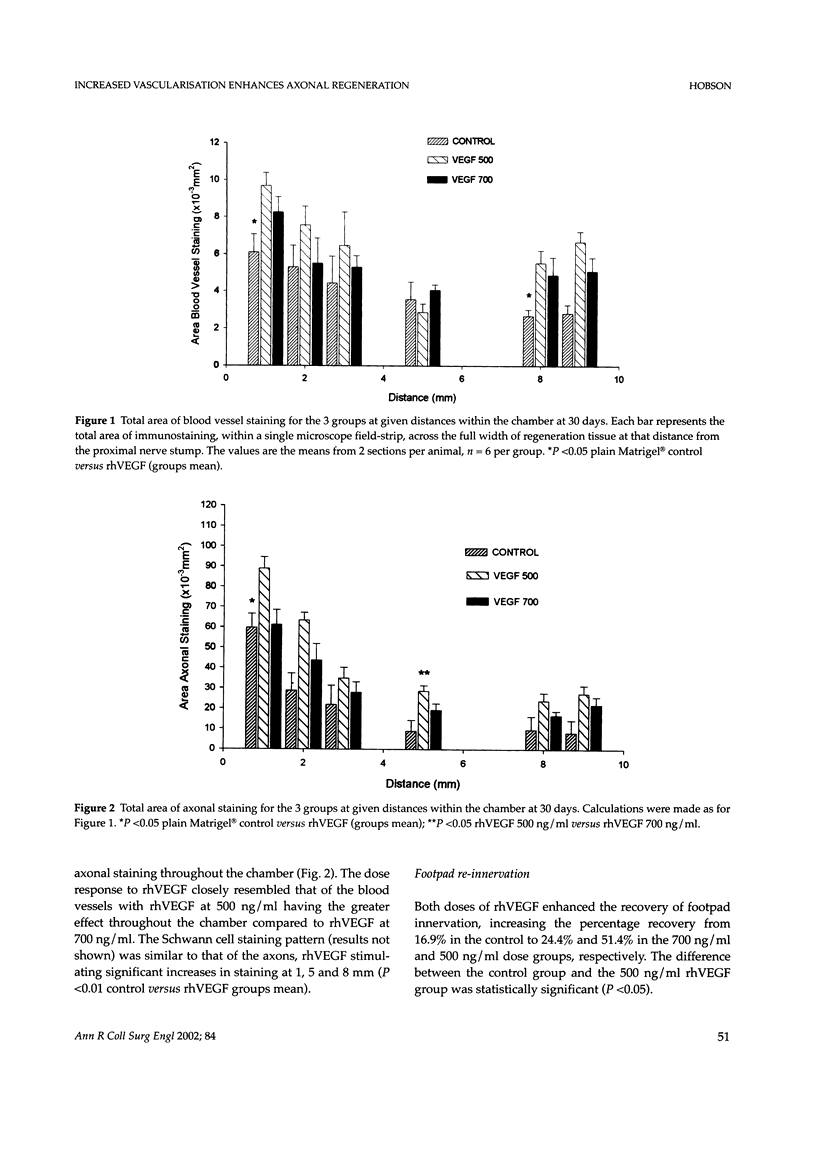
Despite major advances in microsurgical techniques, the functional results of periphery nerve repair remain largely unsatisfactory. Furthermore, if a defect exists such that a nerve graft is required, the results are generally worse than those following a primary repair. The autologous nerve graft, the current 'gold standard', has inherent disadvantages and there has been a long quest to find a suitable alternative. The role of vascularisation in nerve regeneration is poorly defined and the aim of this work was to define and quantify the effects of increased vascularisation on nerve regeneration. Rat sciatic nerve defects (1 cm) were bridged with a silicone chamber containing vascular endothelial growth factor (VEGF; 500 or 700 ng/ml) in a laminin-based gel (Matrigel) known to support axonal regeneration. Chambers were harvested between 5 and 180 days to follow the progression of neural and vascular elements. Following immunohistochemical staining, computerised image analysis demonstrated that VEGF significantly increased vascular, Schwann cell and axonal regeneration within the chamber up to 30 days post-insertion, and stimulated regeneration of up to 78% more myelinated axons at 180 days, compared to plain Matrigel control. Furthermore, the non-linear vascular dose-response to VEGF was clearly reflected in the Schwann cell and axonal staining intensity, supporting the highly significant relationship between vascularisation and Schwann cell staining seen within the chamber (P <0.001). Target-organ re-innervation at 180 days was similarly enhanced by VEGF in an identical dose-dependant manner. VEGF at 500 ng/ml increased recovery of gastrocnemius muscle weight by 17% and footpad innervation by 51% (P <0.05) compared to control, indicating the long-term functional benefits of VEGF.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best T. J., Mackinnon S. E. Peripheral nerve revascularization: a current literature review. J Reconstr Microsurg. 1994 May;10(3):193–204. doi: 10.1055/s-2007-1006587. [DOI] [PubMed] [Google Scholar]
- Borg J. P., deLapeyrière O., Noguchi T., Rottapel R., Dubreuil P., Birnbaum D. Biochemical characterization of two isoforms of FLT4, a VEGF receptor-related tyrosine kinase. Oncogene. 1995 Mar 2;10(5):973–984. [PubMed] [Google Scholar]
- Fawcett J. W., Keynes R. J. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Fields R. D., Le Beau J. M., Longo F. M., Ellisman M. H. Nerve regeneration through artificial tubular implants. Prog Neurobiol. 1989;33(2):87–134. doi: 10.1016/0301-0082(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Gitay-Goren H., Soker S., Vlodavsky I., Neufeld G. The binding of vascular endothelial growth factor to its receptors is dependent on cell surface-associated heparin-like molecules. J Biol Chem. 1992 Mar 25;267(9):6093–6098. [PubMed] [Google Scholar]
- Grant D. S., Kleinman H. K., Goldberg I. D., Bhargava M. M., Nickoloff B. J., Kinsella J. L., Polverini P., Rosen E. M. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson M. I., Brown R., Green C. J., Terenghi G. Inter-relationships between angiogenesis and nerve regeneration: a histochemical study. Br J Plast Surg. 1997 Feb;50(2):125–131. doi: 10.1016/s0007-1226(97)91325-4. [DOI] [PubMed] [Google Scholar]
- Jabaley M. E., Burns J. E., Orcutt B. S., Bryant M. Comparison of histologic and functional recovery after peripheral nerve repair. J Hand Surg Am. 1976 Sep;1(2):119–130. doi: 10.1016/s0363-5023(76)80005-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kuffler D. P. Promoting and directing axon outgrowth. Mol Neurobiol. 1994 Aug-Dec;9(1-3):233–243. doi: 10.1007/BF02816122. [DOI] [PubMed] [Google Scholar]
- Le Beau J. M., Ellisman M. H., Powell H. C. Ultrastructural and morphometric analysis of long-term peripheral nerve regeneration through silicone tubes. J Neurocytol. 1988 Apr;17(2):161–172. doi: 10.1007/BF01674203. [DOI] [PubMed] [Google Scholar]
- Madison R. D., da Silva C., Dikkes P., Sidman R. L., Chiu T. H. Peripheral nerve regeneration with entubulation repair: comparison of biodegradeable nerve guides versus polyethylene tubes and the effects of a laminin-containing gel. Exp Neurol. 1987 Feb;95(2):378–390. doi: 10.1016/0014-4886(87)90146-4. [DOI] [PubMed] [Google Scholar]
- Madison R., da Silva C. F., Dikkes P., Chiu T. H., Sidman R. L. Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and a laminin-containing gel. Exp Neurol. 1985 Jun;88(3):767–772. doi: 10.1016/0014-4886(85)90087-1. [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Nicosia R. F., Ottinetti A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol. 1990 Feb;26(2):119–128. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Goldberg I. D. Scatter factor and angiogenesis. Adv Cancer Res. 1995;67:257–279. doi: 10.1016/s0065-230x(08)60715-0. [DOI] [PubMed] [Google Scholar]
- Shen H., Clauss M., Ryan J., Schmidt A. M., Tijburg P., Borden L., Connolly D., Stern D., Kao J. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood. 1993 May 15;81(10):2767–2773. [PubMed] [Google Scholar]
- Sondell M., Lundborg G., Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999 Jul 15;19(14):5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996 Jan 12;271(2):603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Van Beek A. L., Jacobs S. C., Zook E. G. Examination of peripheral nerves with the scanning electron microscope. Plast Reconstr Surg. 1979 Apr;63(4):509–519. doi: 10.1097/00006534-197904000-00012. [DOI] [PubMed] [Google Scholar]
- Weddell G. Axonal regeneration in cutaneous nerve plexuses. J Anat. 1942 Oct;77(Pt 1):49–62.3. doi: 10.1093/oxfordjournals.bmb.a070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J., May R., Schröder J. M. Fine structural and immunohistochemical identification of perineurial cells connecting proximal and distal stumps of transected peripheral nerves at early stages of regeneration in silicone tubes. Acta Neuropathol. 1994;88(2):159–165. doi: 10.1007/BF00294509. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Longo F. M., Powell H. C., Lundborg G., Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983 Aug 20;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]


