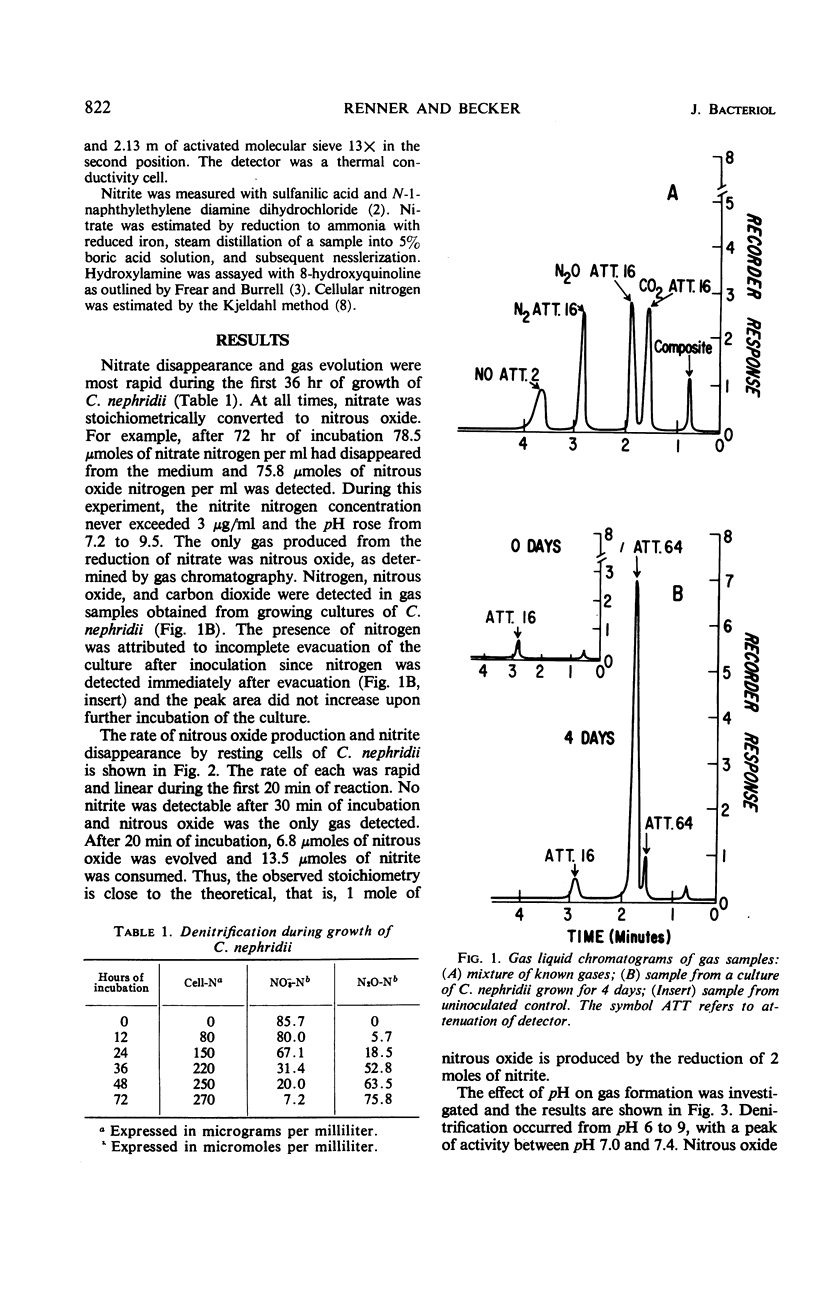
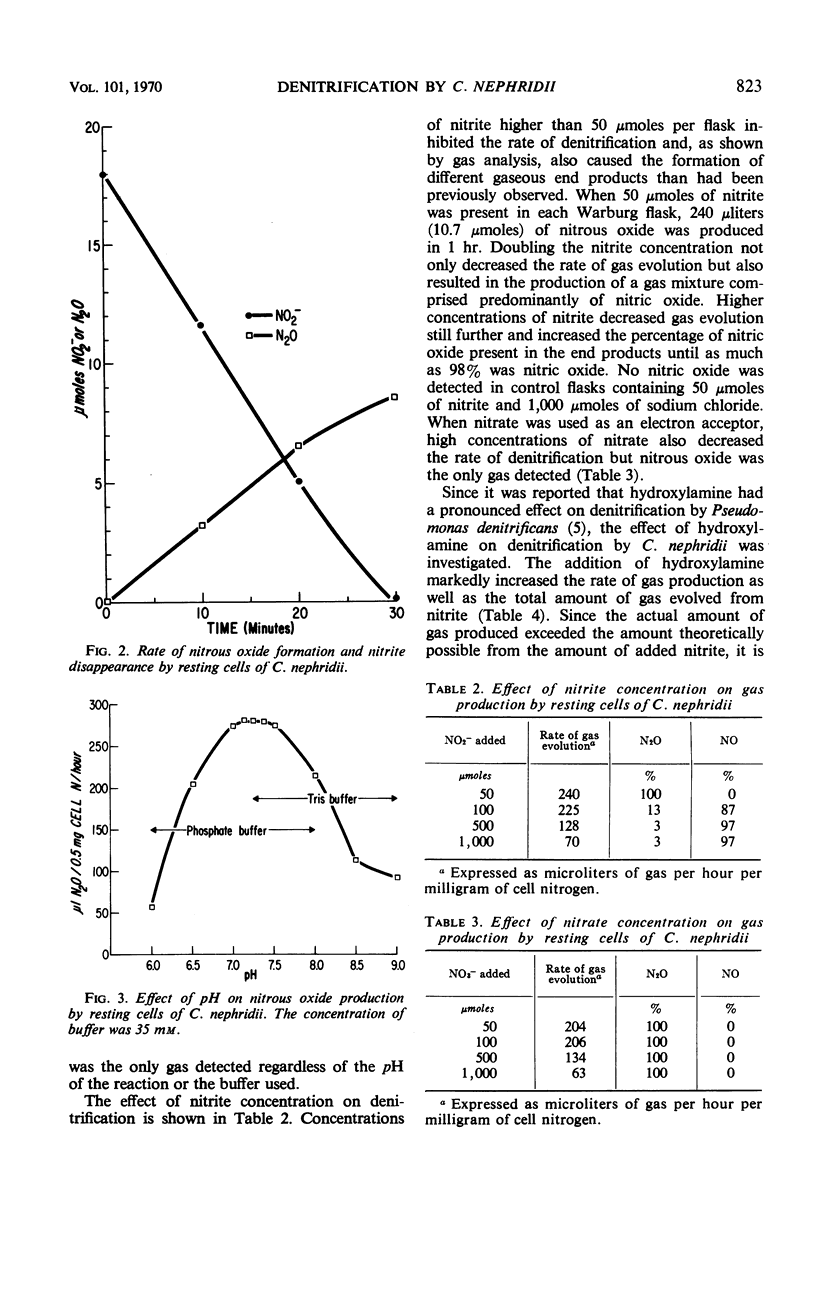
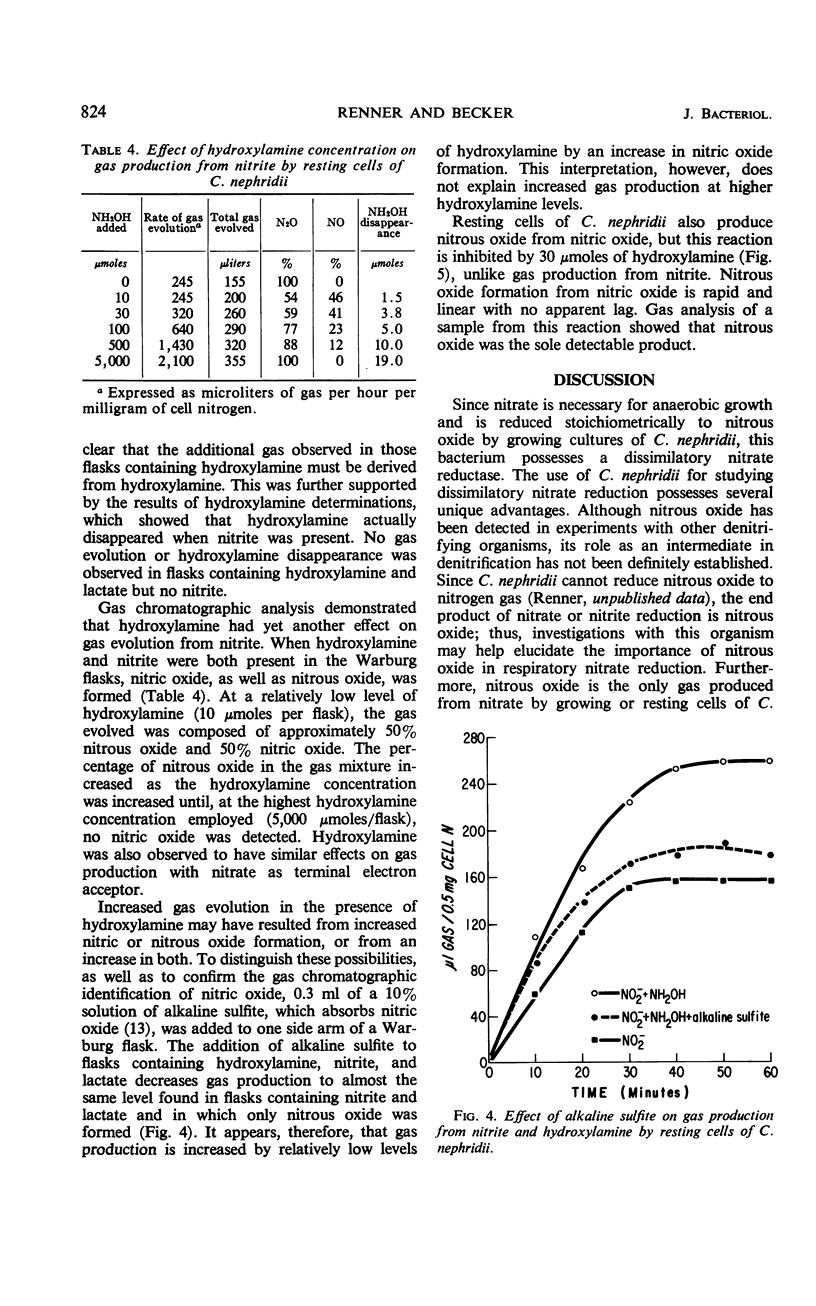
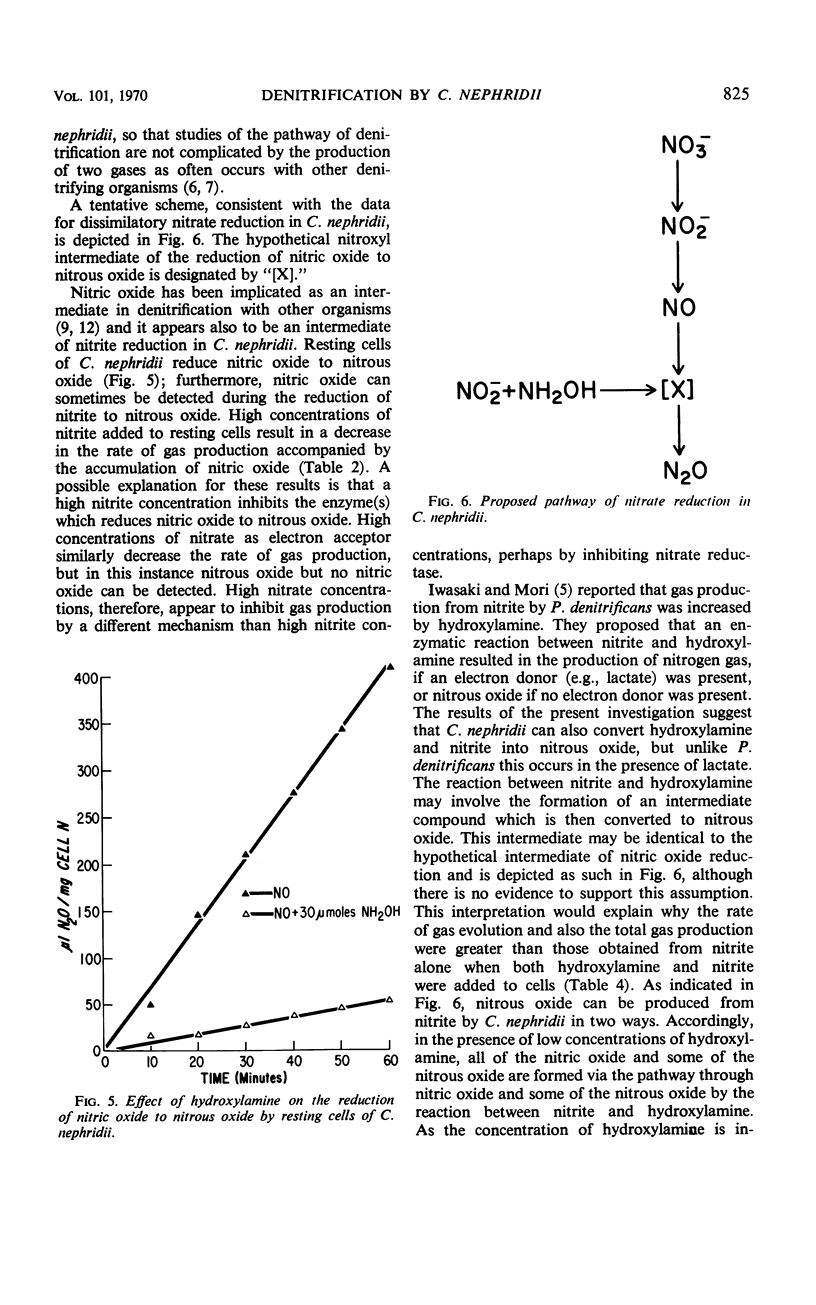
Abstract
Resting cells of Corynebacterium nephridii reduce nitrate, nitrite, and nitric oxide to nitrous oxide under anaerobic conditions. Nitrous oxide production from nitrite was optimal from pH 7.0 to 7.4. The stoichiometry of nitrous oxide production from nitrite was 99% of the theoretical—two moles of nitrite was used for each mole of nitrous oxide detected. Hydroxylamine increases gas evolution from nitrite but inhibits the reduction of nitric oxide to nitrous oxide. Hydroxylamine is converted to nitrogenous gas(es) by resting cells only in the presence of nitrite. Under certain conditions nitric oxide, as well as nitrous oxide, was detected.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B., VAN NIEL C. B. Experiments on bacterial denitrification. J Bacteriol. 1952 Sep;64(3):397–412. doi: 10.1128/jb.64.3.397-412.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART L. T., LARSON A. D., MCCLESKEY C. S. DENITRIFICATION BY CORYNEBACTERIUM NEPHRIDII. J Bacteriol. 1965 Apr;89:1104–1108. doi: 10.1128/jb.89.4.1104-1108.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUYVER A. J., VERHOEVEN W. Studies on true dissimilatory nitrate reduction. II. The mechanism of denitrification. Antonie Van Leeuwenhoek. 1954;20(3):241–262. doi: 10.1007/BF02543727. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Mori T. Studies on denitrification. IX. Nitrous oxide, its production and reduction to nitrogen. J Biochem. 1968 Dec;64(6):863–871. doi: 10.1093/oxfordjournals.jbchem.a128968. [DOI] [PubMed] [Google Scholar]
- Radcliffe B. C., Nicholas D. J. Some properties of a nitrite reductase from Pseudomonas denitrificans. Biochim Biophys Acta. 1968 Apr 2;153(3):545–554. doi: 10.1016/0005-2728(68)90184-9. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BARKER H. A. Substrate oxidation and nitrous oxide utilization in denitrification. J Bacteriol. 1952 Aug;64(2):247–252. doi: 10.1128/jb.64.2.247-252.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERHOEVEN W., GOOS J. J. Studies on true dissimilatory nitrate reduction. I. Fate of the hydrogen donator in bacterial nitrate reduction. Antonie Van Leeuwenhoek. 1954;20(1):93–101. doi: 10.1007/BF02543711. [DOI] [PubMed] [Google Scholar]
- WALKER G. C., NICHOLAS D. J. Nitrite reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:350–360. doi: 10.1016/0006-3002(61)90134-2. [DOI] [PubMed] [Google Scholar]
- WALTERS C. L., TAYLOR A. M. THE IDENTIFICATION AND ESTIMATION OF NITRIC OXIDE BY ITS ABSORPTION IN ALKALINE SODIUM SULPHITE. Biochim Biophys Acta. 1964 Feb 10;82:423–425. doi: 10.1016/0304-4165(64)90321-6. [DOI] [PubMed] [Google Scholar]


