Abstract
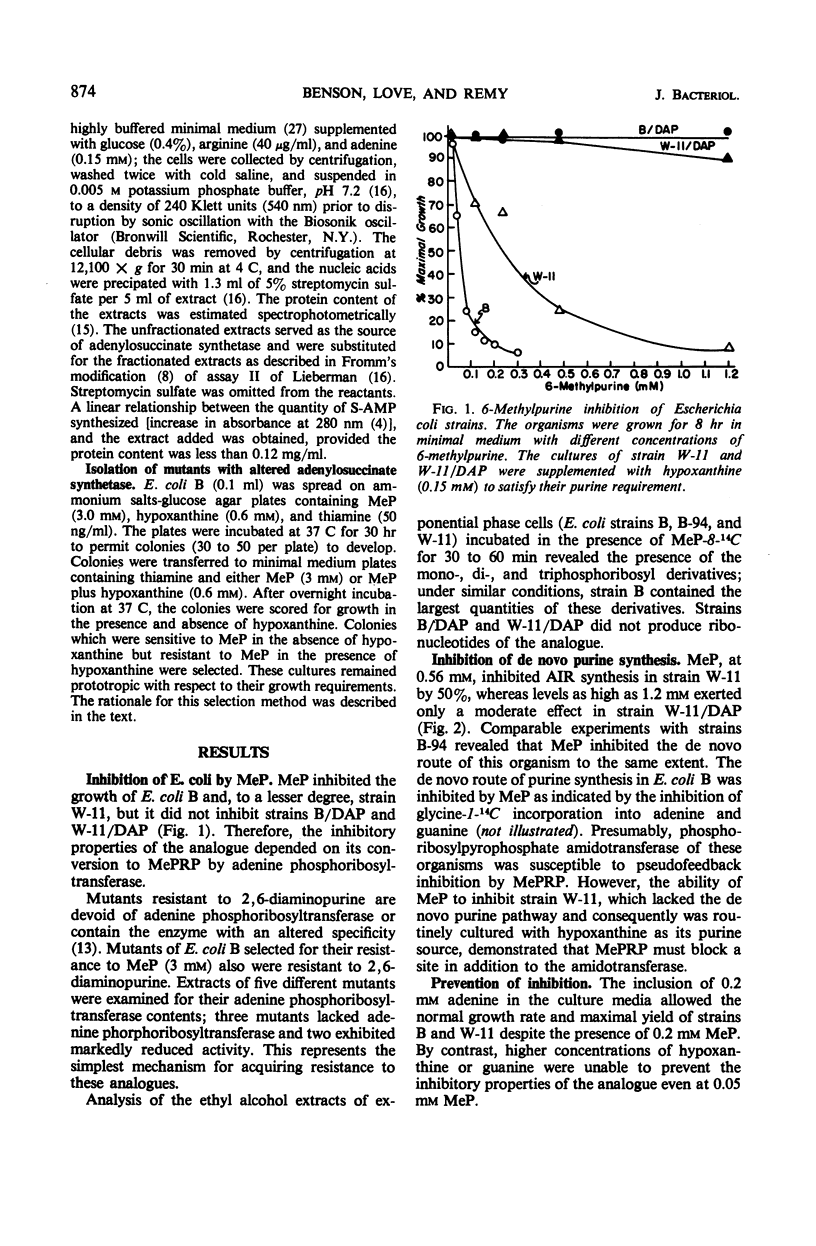
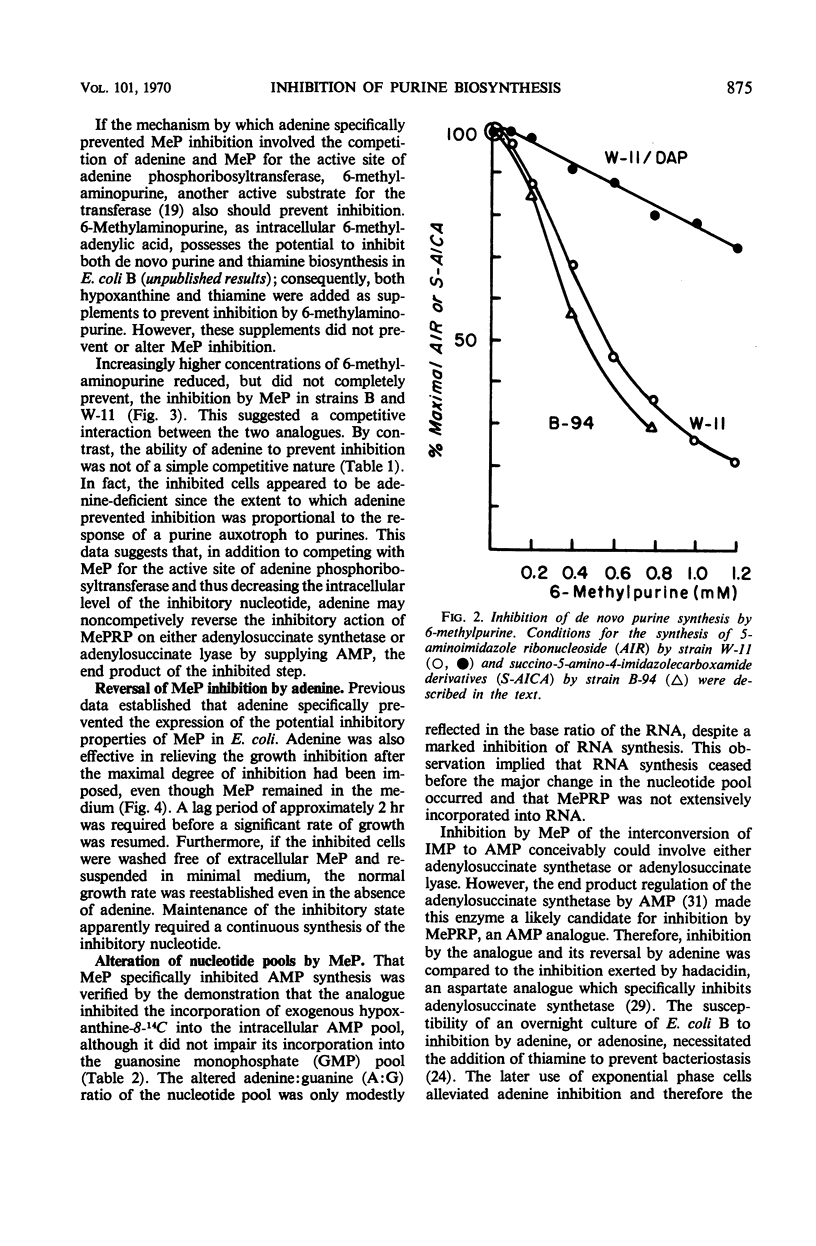
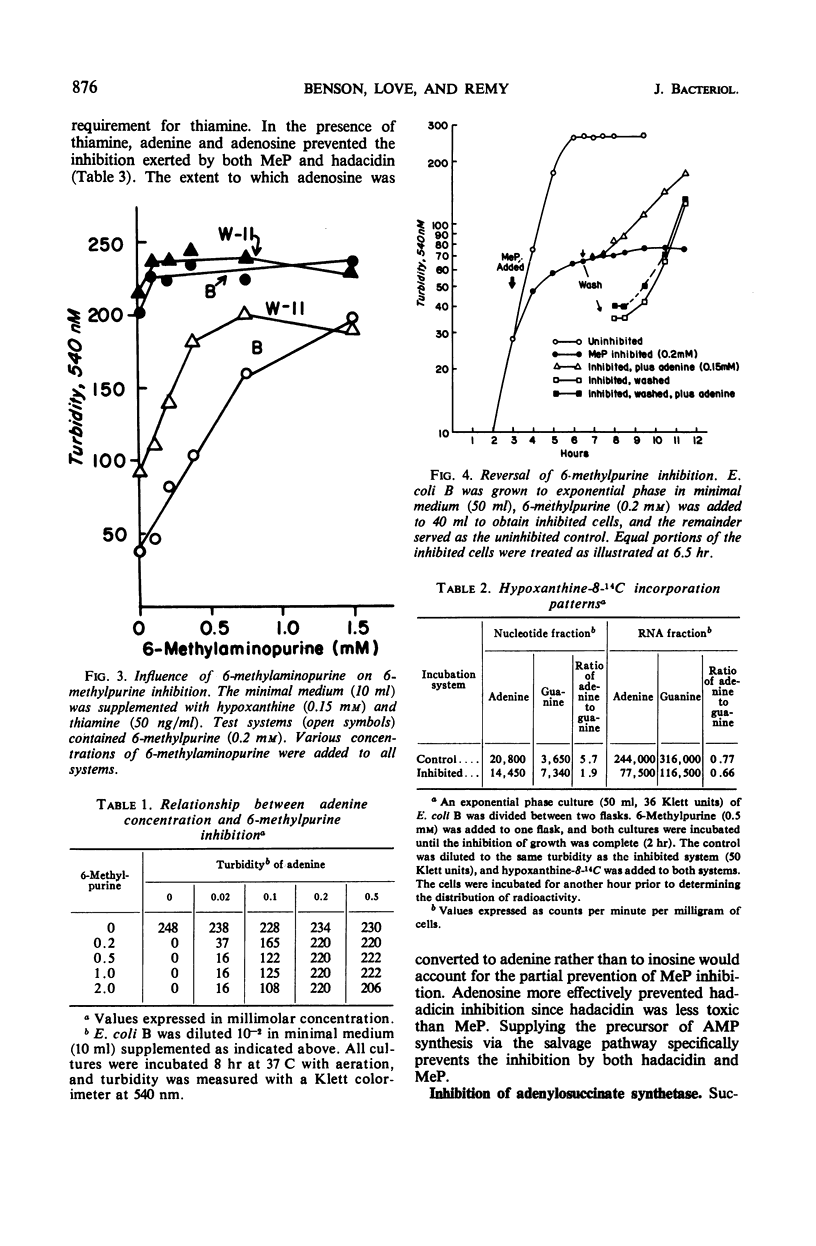
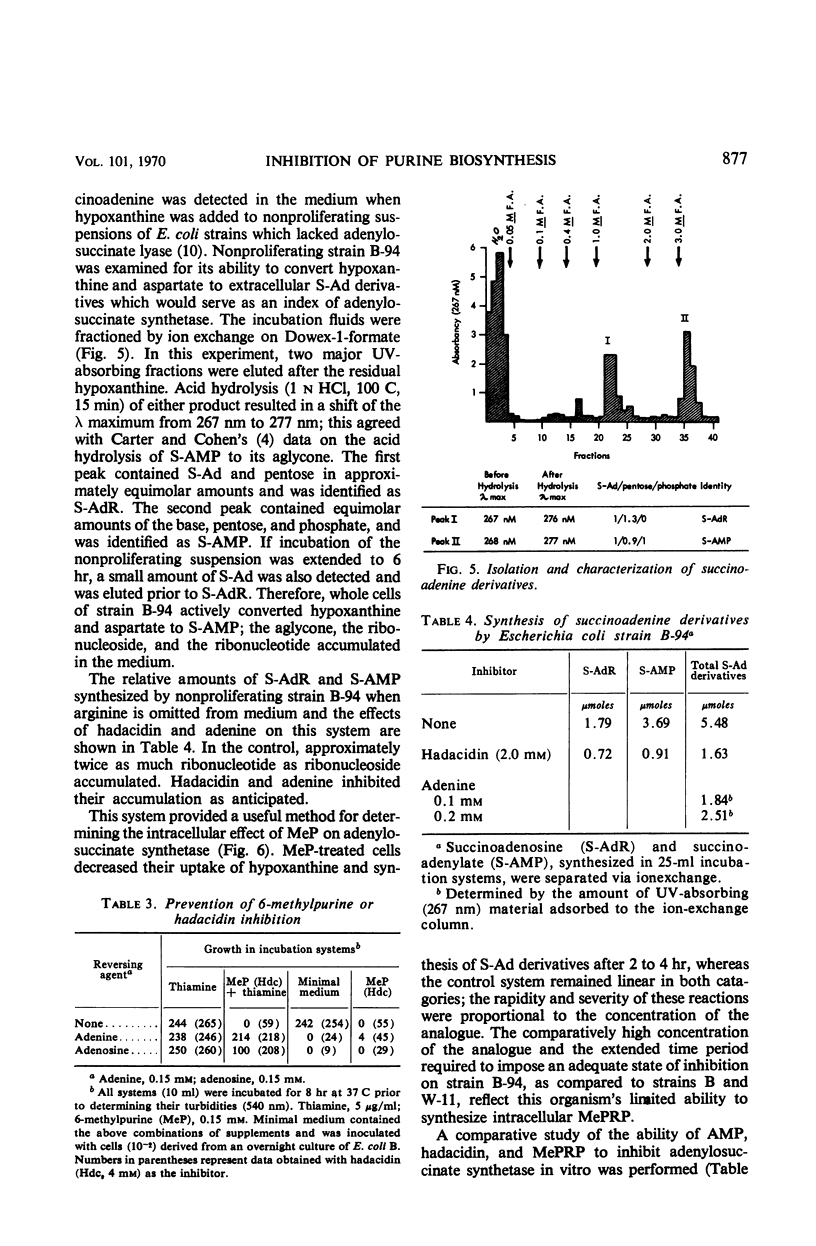
The inhibition of Escherichia coli strain B and strain W-11 by 6-methylpurine depended on the formation of 6-methylpurine ribonucleotide by the action of adenine phosphoribosyltransferase (AMP: pyrophosphate phosphoribosyltransferase, EC 2.4.2.7). 6-Methylpurine ribonucleotide inhibited the de novo synthesis of purines, presumably via pseudofeedback inhibition of phosphoribosylpyrophosphate amidotransferase (EC 2.4.2.14). The same mechanism accounted for its inhibition of adenylosuccinate synthetase [IMP: l-aspartate ligase (GDP), EC 6.3.4.4]. Adenine and 6-methylaminopurine prevented inhibition by competing for the action of adenine phosphoribosyltransferase. In addition, adenine reversed this inhibition by replenishing the AMP to bypass both sites of inhibition. Nonproliferating suspensions of strain B-94, which lacked adenylosuccinate lyase (EC 4.3.2.2), converted exogenous hypoxanthine and aspartate to succinoadenine derivatives which accumulated in the medium. Compounds which inhibited adenylosuccinate synthetase inhibited accumulation of the succinoadenine derivatives. A method was described for the isolation of mutants which potentially possessed an altered adenylosuccinate synthetase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair D. G., Rainnie D. J. Acid-soluble metabolites of 2,6-diaminopurine in L strain mouse cells. Can J Biochem. 1967 Feb;45(2):347–350. doi: 10.1139/o67-040. [DOI] [PubMed] [Google Scholar]
- CARTER C. E., COHEN L. H. The preparation and properties of adenylosuccinase and adenylosuccinic acid. J Biol Chem. 1956 Sep;222(1):17–30. [PubMed] [Google Scholar]
- CARTER C. E. Synthesis of 6-succinoaminopurine. J Biol Chem. 1956 Nov;223(1):139–146. [PubMed] [Google Scholar]
- CLARKE D. A., ELION G. B., HITCHINGS G. H., STOCK C. C. Structure-activity relationships among purines related to 6-mercaptopurine. Cancer Res. 1958 May;18(4):445–456. [PubMed] [Google Scholar]
- DEWEY V. C., HEINRICH M. R., MARKEES D. G., KIDDER G. W. Multiple inhibition by 6-methylpurine. Biochem Pharmacol. 1960 Jun;3:173–180. doi: 10.1016/0006-2952(60)90104-0. [DOI] [PubMed] [Google Scholar]
- FROMM H. J. On the equilibrium and mechanism of adenylosuccinic acid synthesis. Biochim Biophys Acta. 1958 Aug;29(2):255–262. doi: 10.1016/0006-3002(58)90182-3. [DOI] [PubMed] [Google Scholar]
- GOTS J. S., LOVE S. H. Purine metabolism in bacteria. II. Factors influencing biosynthesis of 4-amino-5-imidazolecarboxamide by Escherichia coli. J Biol Chem. 1954 Sep;210(1):395–405. [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gots J. S., Gollub E. G. SEQUENTIAL BLOCKADE IN ADENINE BIOSYNTHESIS BY GENETIC LOSS OF AN APPARENT BIFUNCTIONAL DEACYLASE. Proc Natl Acad Sci U S A. 1957 Sep 15;43(9):826–834. doi: 10.1073/pnas.43.9.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. Alterations in purine nucleotide pyrophosphorylases and resistance to purine analogues. Biochim Biophys Acta. 1961 Oct 14;53:166–173. doi: 10.1016/0006-3002(61)90803-4. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I. Enzymatic synthesis of adenosine-5'-phosphate from inosine-5'-phosphate. J Biol Chem. 1956 Nov;223(1):327–339. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A., SIMMS E. S. Enzymatic synthesis of pyrimidine nucleotides; orotidine-5'-phosphate and uridine-5'-phosphate. J Biol Chem. 1955 Jul;215(1):403–451. [PubMed] [Google Scholar]
- LOVE S. H., GOTS J. S. Purine metabolism in bacteria. III. Accumulation of a new pentose-containing arylamine by a purine-requiring mutant of Escherichia coli. J Biol Chem. 1955 Feb;212(2):647–654. [PubMed] [Google Scholar]
- Lallier R. Effets du 5-fluoro-uracile et de la 6-méthylpurine sur le développment de l'oeuf de l'oursin Paracentrotus lividus. J Embryol Exp Morphol. 1965 Oct;14(2):181–189. [PubMed] [Google Scholar]
- Love S. H., Remy C. N. Metabolism of methylated purines in Escherichia coli: derepression of purine biosynthesis. J Bacteriol. 1966 Mar;91(3):1037–1049. doi: 10.1128/jb.91.3.1037-1049.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. Chromatographic studies of nucleic acids; a technique for the identification and estimation of purine and pyrimidine bases, nucleosides and related substances. Biochem J. 1949;45(3):294–298. doi: 10.1042/bj0450294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. H., KEMPNER E. S. EFFECTS OF AN ADENINE ANALOG ON YEAST METABOLISM. Biochim Biophys Acta. 1963 Nov 22;76:333–340. [PubMed] [Google Scholar]
- MOYED H. S. INHIBITION OF THE BIOSYNTHESIS OF THE PYRIMIDINE PORTION OF THIAMINE BY ADENOSINE. J Bacteriol. 1964 Oct;88:1024–1029. doi: 10.1128/jb.88.4.1024-1029.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Properties of a bacterial mutant lacking amino acid control of RNA synthesis. Biochim Biophys Acta. 1963 Mar 26;68:365–379. doi: 10.1016/0006-3002(63)90158-6. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Tucker R. G. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem J. 1968 Jan;106(1):279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMY C. N., SMITH M. S. Metabolism of 2, 6-diaminopurine; conversion to 5'-phosphoribosyl-2-methylamino-beta-aminopurine by enzymes of Escherichia coli. J Biol Chem. 1957 Sep;228(1):325–338. [PubMed] [Google Scholar]
- Remy C. N. Ribonucleotides and ribonucleosides as methyl acceptors for S-adenosylmethionine: (amino- and thio-)purine methyl-transferases. Incorporation of 6-amino-2-methylaminopurine into ribonucleic acids. Biochim Biophys Acta. 1967 Apr 18;138(2):258–275. [PubMed] [Google Scholar]
- SHIGEURA H. T., GORDON C. N. The mechanism of action of hadacidin. J Biol Chem. 1962 Jun;237:1937–1940. [PubMed] [Google Scholar]
- WYNGAARDEN J. B., GREENLAND R. A. The inhibition of succinoadenylate kinosynthetase of Escherichia coli by adenosine and guanosine 5'-monophosphates. J Biol Chem. 1963 Mar;238:1054–1057. [PubMed] [Google Scholar]
- ZIMMERMAN E. F., MAGASANIK B. UTILIZATION AND INTERCONVERSION OF PURINE BASES AND RIBONUCLEOSIDES BY SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jan;239:293–300. [PubMed] [Google Scholar]


