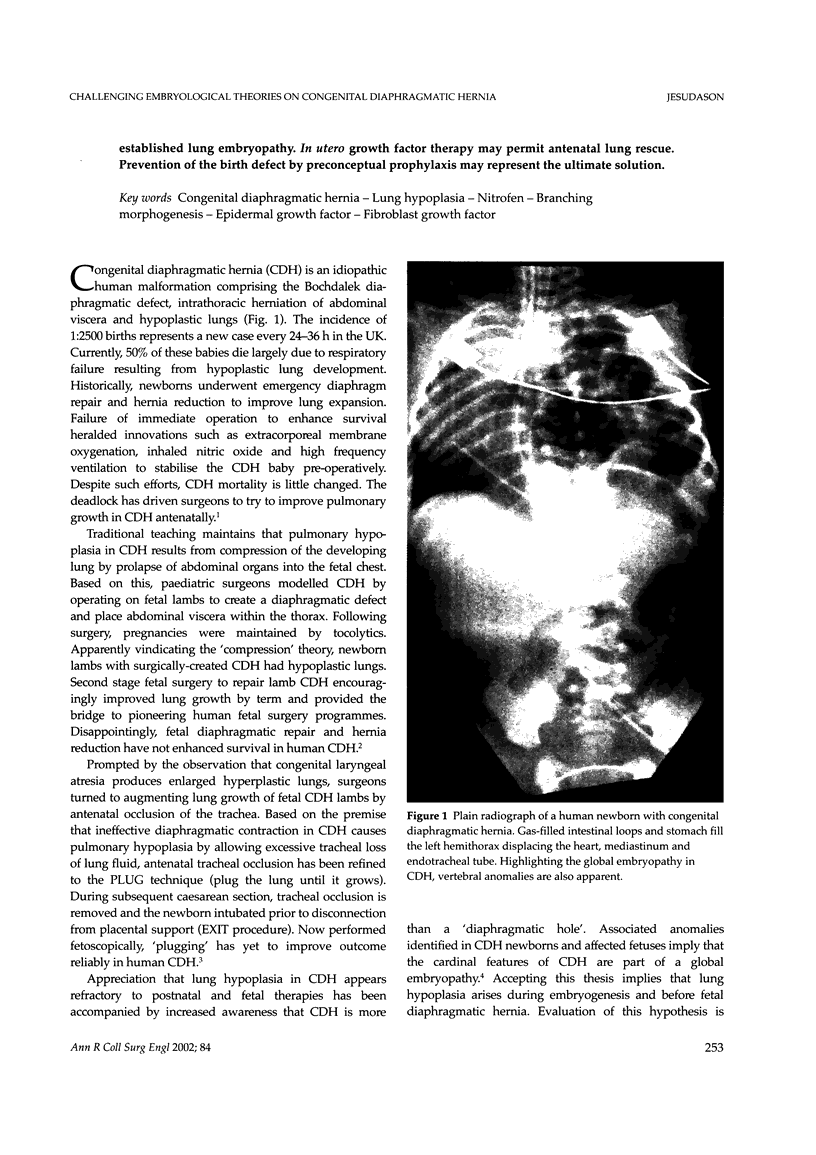
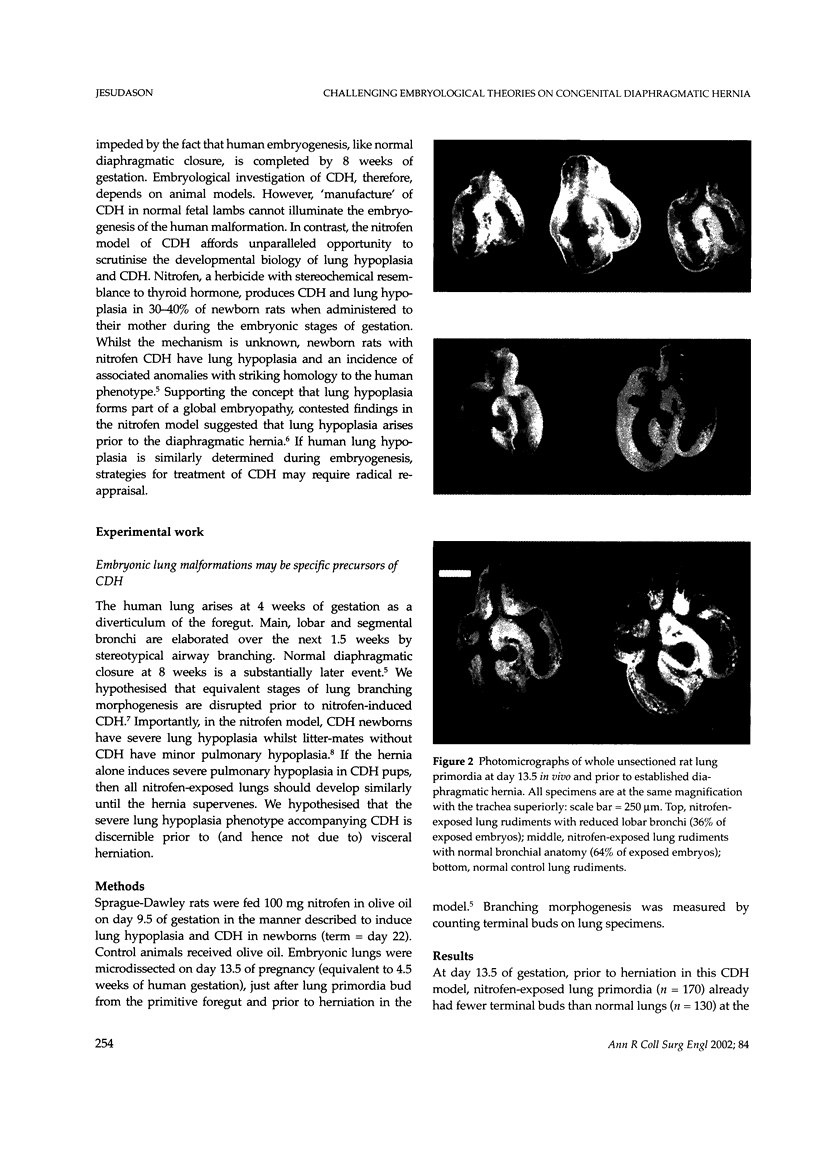
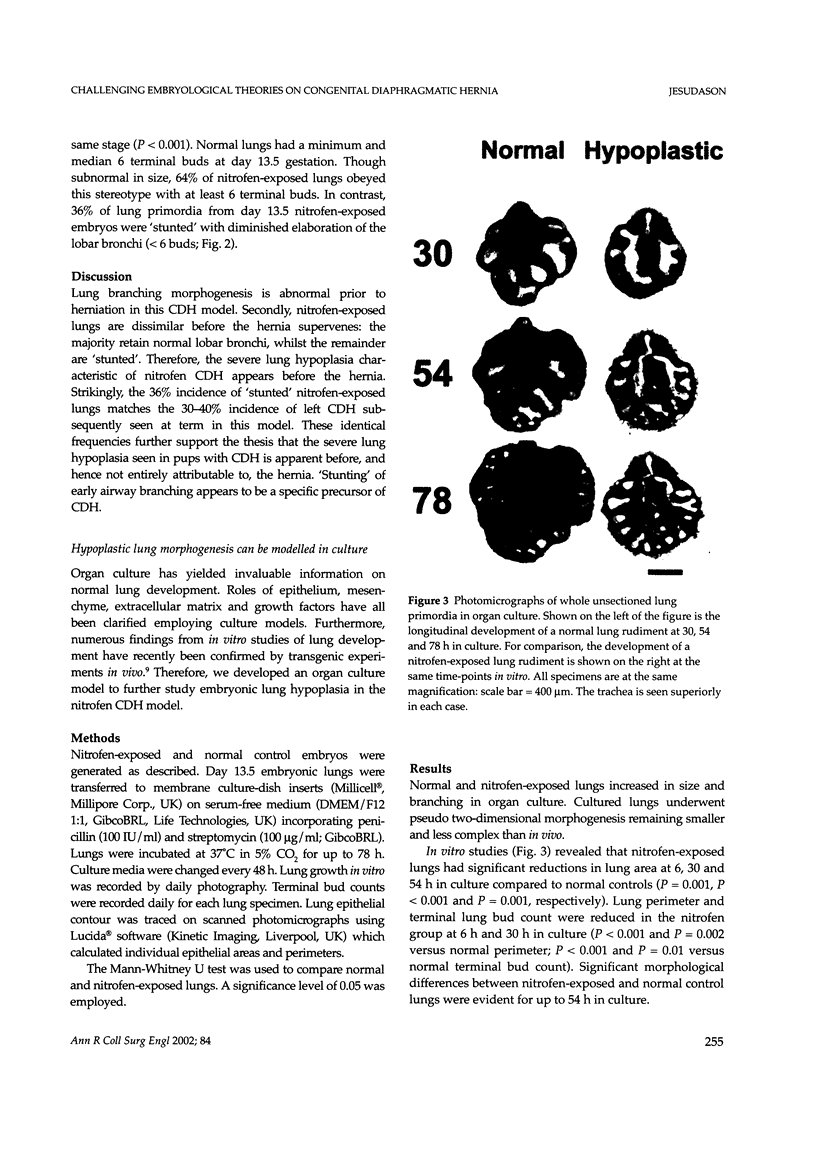
Abstract
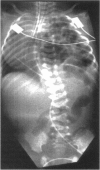
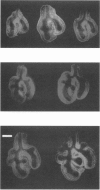
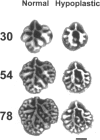
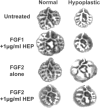
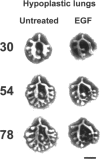
Lung hypoplasia is central to the poor prognosis of babies with congenital diaphragmatic hernia (CDH). Prolapse of abdominal organs through a diaphragmatic defect has traditionally been thought to impair lung growth by compression. The precise developmental biology of CDH remains unresolved. Refractory to fetal correction, lung hypoplasia in CDH may instead originate during embryogenesis and before visceral herniation. Resolving these conflicting hypotheses may lead to reappraisal of current clinical strategies. Genetic studies in murine models and the fruitfly, Drosophila melanogaster are elucidating the control of normal respiratory organogenesis. Branchless and breathless are Drosophila mutants lacking fibroblast growth factor (FGF) and its cognate receptor (FGFR), respectively. Sugarless and sulphateless mutants lack enzymes essential for heparan sulphate (HS) biosynthesis. Phenotypically, all these mutants share abrogated airway branching. Mammalian organ culture and transgenic models confirm the essential interaction of FGFs and HS during airway ramification. Embryonic airway development (branching morphogenesis) occurs in a defined spatiotemporal sequence. Unlike the surgically-created lamb model, the nitrofen rat model permits investigation of embryonic lung growth in CDH. Microdissecting embryonic lung primordia from the nitrofen CDH model and normal controls, we demonstrated that disruption of stereotyped airway branching correlates with and precedes subsequent CDH formation. To examine disturbed branching morphogenesis longitudinally, we characterised a system that preserves lung hypoplasia in organ culture. We tested FGFs and heparin (an HS analogue) as potential therapies on normal and hypoplastic lungs. Observing striking differences in morphological response to FGFs between normal and hypoplastic lung primordia, we postulated abnormalities of FGF/HS signalling in the embryonic CDH lung. Evaluating this hypothesis further, we examined effects of an HS-independent growth factor (epidermal growth factor, EGF) on hypoplastic lung development. Visible differences in morphological response indicate an intrinsic abnormality of hypoplastic lung primordia that may involve shared targets of FGFs and EGE. These studies indicate that lung hypoplasia precedes diaphragmatic hernia and may involve disturbances of mitogenic signalling pathways fundamental to embryonic lung development. What does this imply for human CDH? Fetal surgery may be 'too little, too late' to correct an established lung embryopathy. In utero growth factor therapy may permit antenatal lung rescue. Prevention of the birth defect by preconceptual prophylaxis may represent the ultimate solution.
Full text
PDF

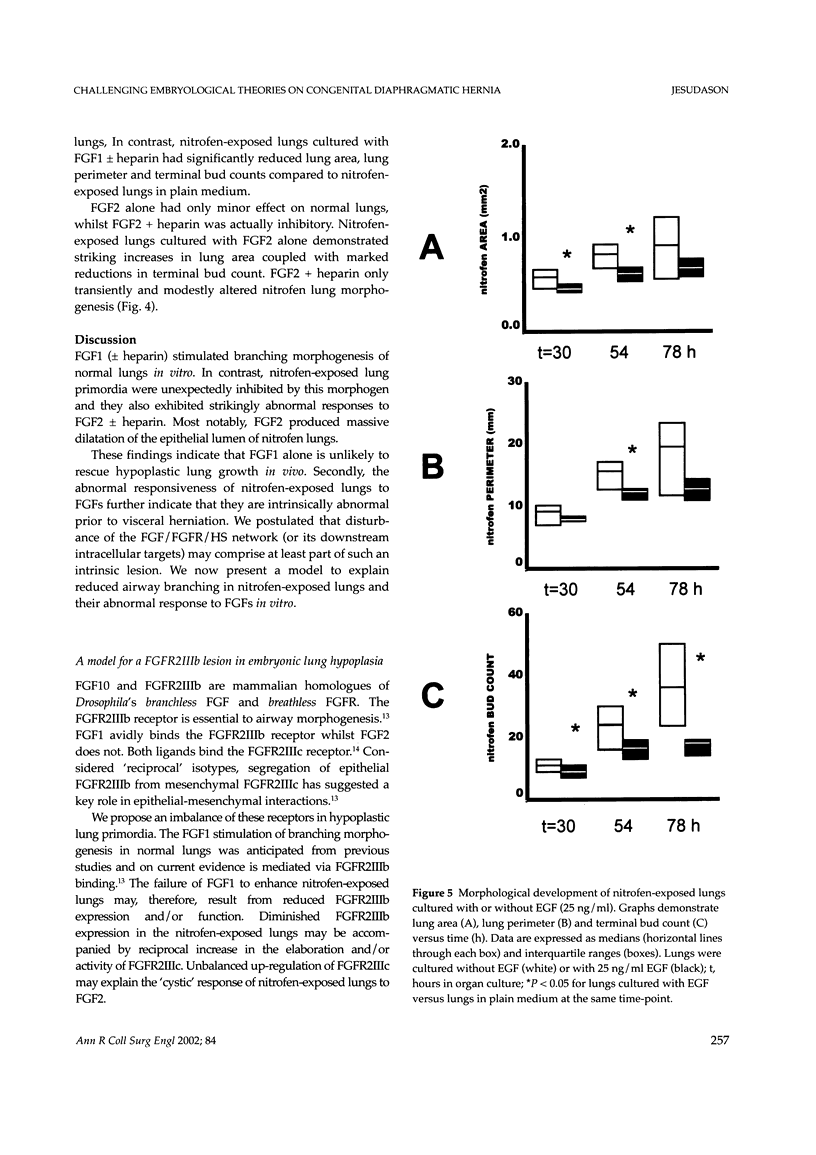
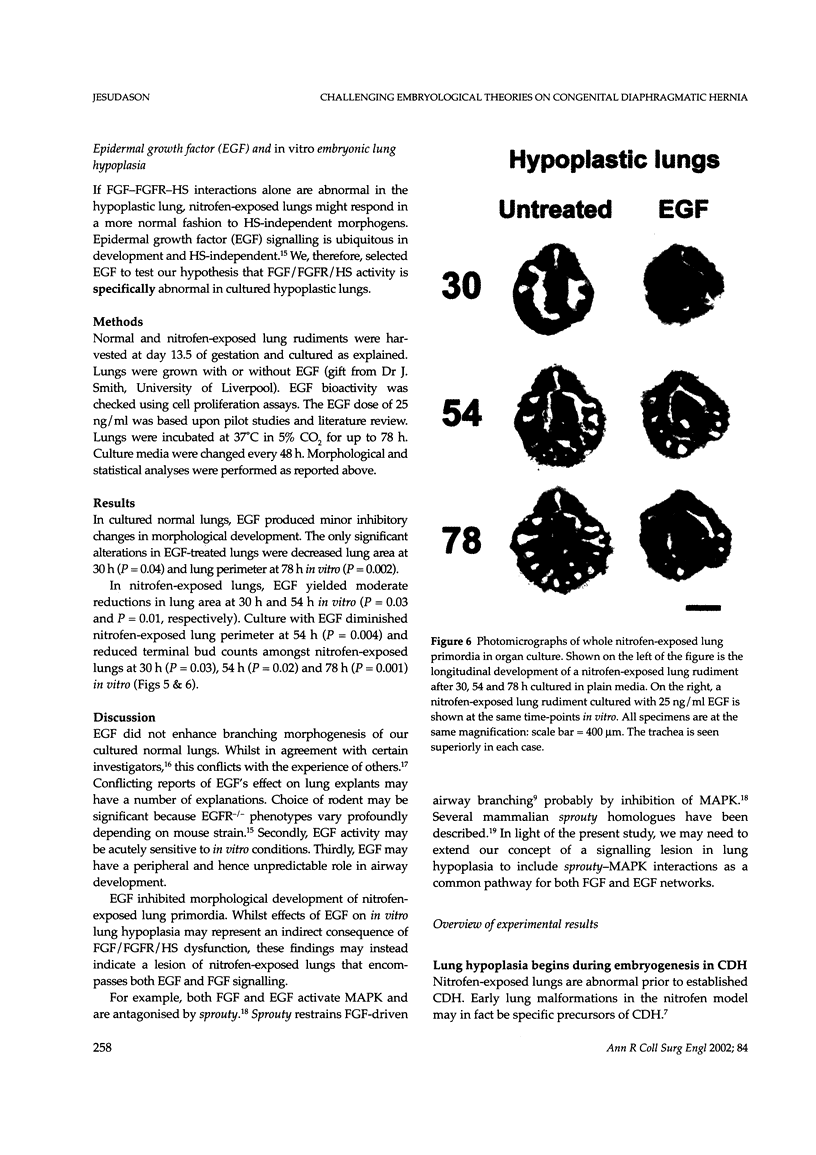

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzick N. S., Nance M. L. Pediatric surgery. First of two parts. N Engl J Med. 2000 Jun 1;342(22):1651–1657. doi: 10.1056/NEJM200006013422207. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L., Spencer-Dene B., Revest J. M., Hajihosseini M., Rosewell I., Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000 Feb;127(3):483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Ganser G. L., Stricklin G. P., Matrisian L. M. EGF and TGF alpha influence in vitro lung development by the induction of matrix-degrading metalloproteinases. Int J Dev Biol. 1991 Dec;35(4):453–461. [PubMed] [Google Scholar]
- Harrison M. R., Adzick N. S., Bullard K. M., Farrell J. A., Howell L. J., Rosen M. A., Sola A., Goldberg J. D., Filly R. A. Correction of congenital diaphragmatic hernia in utero VII: a prospective trial. J Pediatr Surg. 1997 Nov;32(11):1637–1642. doi: 10.1016/s0022-3468(97)90472-3. [DOI] [PubMed] [Google Scholar]
- Iritani I. Experimental study on embryogenesis of congenital diaphragmatic hernia. Anat Embryol (Berl) 1984;169(2):133–139. doi: 10.1007/BF00303142. [DOI] [PubMed] [Google Scholar]
- Jesudason E. C., Connell M. G., Fernig D. G., Lloyd D. A., Losty P. D. Early lung malformations in congenital diaphragmatic hernia. J Pediatr Surg. 2000 Jan;35(1):124–128. doi: 10.1016/s0022-3468(00)80028-7. [DOI] [PubMed] [Google Scholar]
- Jesudason E. C., Connell M. G., Fernig D. G., Lloyd D. A., Losty P. D. Heparin and in-vitro experimental lung hypoplasia. Pediatr Surg Int. 2000;16(4):247–251. doi: 10.1007/s003830050738. [DOI] [PubMed] [Google Scholar]
- Jesudason E. C., Connell M. G., Fernig D. G., Lloyd D. A., Losty P. D. In vitro effects of growth factors on lung hypoplasia in a model of congenital diaphragmatic hernia. J Pediatr Surg. 2000 Jun;35(6):914–922. doi: 10.1053/jpsu.2000.6919. [DOI] [PubMed] [Google Scholar]
- Kitano Y., Adzick N. S. New developments in fetal lung surgery. Curr Opin Pediatr. 1999 Jun;11(3):193–199. doi: 10.1097/00008480-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Kluth D., Keijzer R., Hertl M., Tibboel D. Embryology of congenital diaphragmatic hernia. Semin Pediatr Surg. 1996 Nov;5(4):224–233. [PubMed] [Google Scholar]
- Lin X., Buff E. M., Perrimon N., Michelson A. M. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999 Sep;126(17):3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Losty P. D. Recent advances: paediatric surgery. BMJ. 1999 Jun 19;318(7199):1668–1672. doi: 10.1136/bmj.318.7199.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger R. J., Krasnow M. A. Genetic control of branching morphogenesis. Science. 1999 Jun 4;284(5420):1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Xu J., Colvin J. S., McEwen D. G., MacArthur C. A., Coulier F., Gao G., Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996 Jun 21;271(25):15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Puri P., Gorman F. Lethal nonpulmonary anomalies associated with congenital diaphragmatic hernia: implications for early intrauterine surgery. J Pediatr Surg. 1984 Feb;19(1):29–32. doi: 10.1016/s0022-3468(84)80010-x. [DOI] [PubMed] [Google Scholar]
- Reich A., Sapir A., Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999 Sep;126(18):4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- Tenbrinck R., Tibboel D., Gaillard J. L., Kluth D., Bos A. P., Lachmann B., Molenaar J. C. Experimentally induced congenital diaphragmatic hernia in rats. J Pediatr Surg. 1990 Apr;25(4):426–429. doi: 10.1016/0022-3468(90)90386-n. [DOI] [PubMed] [Google Scholar]
- Threadgill D. W., Dlugosz A. A., Hansen L. A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R. C. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995 Jul 14;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Warburton D., Schwarz M., Tefft D., Flores-Delgado G., Anderson K. D., Cardoso W. V. The molecular basis of lung morphogenesis. Mech Dev. 2000 Mar 15;92(1):55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Warburton D., Seth R., Shum L., Horcher P. G., Hall F. L., Werb Z., Slavkin H. C. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev Biol. 1992 Jan;149(1):123–133. doi: 10.1016/0012-1606(92)90269-m. [DOI] [PubMed] [Google Scholar]