Abstract
Major surgical resection is often the only curative treatment for cholangiocarcinoma. When imaging techniques fail to establish the accurate diagnosis, biopsy of the lesion is unavoidable. However, biopsy is not necessarily required for topography of the cholangiocarcinoma (intrahepatic or extrahepatic). 1) In extrahepatic cholangiocarcinoma (ECC), clinical features and radiological imaging relate to biliary obstruction. Provided that between 8% and 43% of bile duct strictures are not ECC, the lesions mimicking ECC that should be ruled out are gallbladder cancer, Mirizzi syndrome, primary sclerosing cholangitis (PSC), autoimmune pancreatitis and portal biliopathy. Systematic biopsy is usually difficult and has poor sensitivity, but a good knowledge of these mimicking ECC diseases, along with precise analysis of clinical and imaging semiology, may lead to a correct diagnosis without the need for biopsy. 2) Intrahepatic cholangiocarcinoma (ICC) developing in normal liver appears as a hypovascular tumour with fibrotic component and capsular retraction that can be confused with fibrous metastases such as breast and colorectal cancers. The lack of the primary site, a relatively large tumour size and ancillary findings such as bile duct dilatation may provide a clue to the diagnosis. If not, we advocate local resection with lymph node dissection, since ICC is the most likely diagnostis and surgery is the only curative treatment. In the event of adenocarcinoma from unknown primary, surgery is an effective treatment even if prognosis is poor.
Introduction
Radical resection is the only curative treatment of cholangiocarcinoma occurring at any level of the biliary tract, i.e. within the liver (intrahepatic cholangiocarcinoma; ICC) or from extrahepatic bile ducts (extrahepatic cholangiocarcinoma; ECC). Preoperative biopsy of the lesion seems unavoidable when major surgery is planned, which is necessary in most cases.
However, systematic biopsy is often difficult in patients with ECC, but we believe that a good knowledge of diseases mimicking cholangiocarcinoma combined with precise patient semiologic analysis may lead to a correct diagnosis without the need for biopsy. In our experience, and in accordance with a review of the literature, the aim of this article was to describe a preoperative strategy in patients suspected of cholangiocarcinoma.
Extrahepatic cholangiocarcinoma
ECC is generally an infiltrative and sclerosing adenocarcinoma leading to biliary obstruction. It affects men more often than it affects women, and in the age range 50 to 70 years. Clinical presentation of ECC is related to biliary obstruction, i.e. jaundice, dark urine, pale stool and pruritus 1,2. Biochemical examination shows high levels of serum bilirubin, alcalin phosphatases and gamma glutamyl transpeptidase. Cancer Antigen 19-9 (CA19-9) is often elevated but without lack of specificity. Radiological examinations are essential for diagnosis and staging before treatment of ECC 3. CT scan, which reveals intrahepatic bile duct dilatation up to the site of obstruction, assesses vessel encasement, often associated liver atrophy and detects lymphadenopathy. Magnetic resonance cholangiopancreatography (MRCP) refines these findings and allows cholangiography without the risk of cholangitis or pancreatitis. It shows localized strictures, often irregular, bile duct above and below the obstruction, vessel encasement, invasion of adjacent liver parenchyma by hilar cholangiocarcinomas, local lymphadenopathy and distant metastases. However, as indicated in Table I, between 8% and 43% of biliary strictures are not ECC, including malignant strictures other than ECC and benign strictures 4,5,6,7,8,9,10,11,12,13,14. These non-ECC biliary strictures must be researched before patients are referred to long and risky surgical treatment of ECC.
Table I. Studies dealing with biliary strictures mimicking ECC.
| n | ECC | Non-ECC (%) | Gallbladder cancer | Lithiasis | PSC | SSC | Others (malign) | |
|---|---|---|---|---|---|---|---|---|
| Hadjis 1985 8 | 104 | 96 | 8 (17) | 8 | ||||
| Wetter 1991 13 | 98 | 68 | 30 (31) | 12 | 2 | 6 | 10 | |
| Verbeek 1992 12 | 82 | 71 | 11 (13) | 11 | ||||
| Nakayama 1999 10 | 99 | 85 | 14 (14) | 14 | ||||
| Gerhards 2001 7 | 132 | 112 | 20 (15) | 3 | 17 | |||
| Knoefel 2003 14 | 33 | 27 | 6 (18) | 6 | ||||
| Koea 2004 9 | 49 | 28 | 21 (43) | 7 | 2 | 10 | 2 | |
| Corvera 2005 6 | 275 | 253 | 22 (8) | 6 | 3 | 13 | ||
| Are 2006 4 | 171 | 141 | 30 (18) | 16 | 1 | 8 | 5 |
ECC: extrahepatic cholangiocarcinoma; PSC: primary sclerosing cholangitis; SSC: secondary sclerosing cholangitis.
There are several arguments in favour of preoperative biopsy, especially when imaging techniques fail to demonstrate a mass lesion 3. Additionally, surgery can be performed in suitable candidates, and with greater confidence, when there is a positive tissue diagnosis. However, a percutaneous approach with ultrasonography (US) or computed tomography (CT) guidance may fail because of the absence of a visible mass. This approach has also been considered inadvisable because of the possible risk of intra-abdominal seeding of tumour cells 15. It has therefore been proposed that direct methods tissue sampling – either via the biliary duct during endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous cholangiography (PTC), or by a trans-duodenal or trans-gastric route with endoscopic ultrasound (EUS) guidance – may yield better results, with a potentially lower risk of tumour cell spread. Ideally, any tissue sampling technique used should be highly sensitive in detecting cancer, and with absolute specificity. The technique should be simple, safe and relatively inexpensive for widespread used. Unfortunately, none of the currently used tissue sampling methods have all these characteristics. All current methods have relatively low to moderate sensitivity but almost 100% specificity 16.
The main tissue sampling methods during endoscopic procedures are ERCP brush cytology, forceps biopsy and fine-needle aspiration (FNA) and EUS-guided FNA 16. Brushing is the most frequently used tissue sampling technique because it is technically easy, requires little time and is generally safe. Although it has specificity close to 100%, brush cytology is less sensitive in detecting cancer, ranging from 18% to 60% in most published series 17,18,19,20,21,22,23,24,25,26. It has been suggested that this limited sensitivity is at least partially due to failure to obtain an adequate cellular yield. This may be attributed to submucosal tumour growth or to extrinsic location of the tumour with compression of the biliary tree 22,27. Some authors have found that the cancer detection rate of bile cytology increases from 27% to 63% with stricture dilatation 28, and even more using a scraping brush 29. Finally, two consecutives brushings increase the cancer detection rate from 33% (one brushing) to 44% 30. The endobiliary forceps biopsy provides a sample of bile duct tissue deep to the epithelium, theoretically obviating the problem of inadequate sampling that may occur with brushing. This technique is more time-consuming than brushing and is less widely used 16. Sensibility of the technique rises from 43% to 81%, with specificity of around 100% 20,24,25. Several studies have shown that combining several techniques for obtaining tissue samples from biliary strictures at ERCP enhances the detection of cancer. When combining brushing, biopsy and endoscopic FNA, sensibility reaches 70% 20,31,32.
In a recent systematic survey of prospective studies including 16,855 patients, the rate of ERCP-attributable complications was 7%, including pancreatitis (3.5%), sepsis (1.4%), bleeding (1.3%) and perforations (0.6%) 33. Complications directly related to brushing are very rare. One retroperitoneal perforation was reported in a series of 223 consecutive brushings 24, with the rate of post-procedure pancreatitis <2% 34. Complications relating to the use of forceps are uncommon. Minor bleedings have been reported 24.
There are few published data with respect to EUS-FNA of biliary tumours. Recent studies have obtained excellent results, i.e. with 86% to 89% sensitivity 35,36. In one prospective study comparing ERCP and EUS in the diagnosis of biliary strictures, it was concluded that ERCP-based tissue acquisition may be better for biliary tumours, whereas EUS-FNA is preferable for pancreatic mass lesions 37.
Finally, we did not find any cost effectiveness study in the literature about preoperative tissue sampling for cholangiocarcinoma. Thus, other aetiologies of biliary strictures which may mimic ECC must be considered before referring the patient for surgical resection. We advocate that good knowledge of the literature and precise semiologic analysis may lead to the true diagnosis without biopsy.
Malign aetiologies mimicking ECC
1) Carcinoma of the gallbladder. – Although the main location of stricture is usually below the biliary confluence, this diagnosis must be considered systematically. Indeed, it is the most common cause of malignant bile duct stricture in the mid-portion of the common duct. The mechanism can be either invasion or compression. On imaging, diagnosis can be suspected on: (a) presence of an enlarged gallbladder with gallstones, (b) localized gallbladder wall thickening, or (c) invasion of the liver. Occlusion of the cystic duct at endoscopic cholangiography suggests gallbladder carcinoma 38. Anomaly of a pancreaticobiliary duct junction is associated with gallbladder carcinoma in about one-fifth, and must be investigated 39. Finally, patients with carcinoma of gallbladder presenting with jaundice are at particularly high risk of portal vein invasion with poor prognosis because this is not amenable to surgery 40.
2) Lymph node metastases. – Lymph node metastases in the porta hepatis can also cause extrahepatic biliary tree compression. Main causes of lymph node metastasis include colorectal metastases, carcinoma of the breast, lung, stomach, kidney, malignant melanoma and lymphoid neoplasm 41,42,43. Clinical features and imaging can usually make the difference from ECC. Treatment is endoscopic and/or aetiologic.
Benign aetiologies mimicking ECC
Between 8% and 28% of bile duct strictures are of benign origin 4,5,6,7,8,9,10,11,12,13. According to the literature, the following aetiologies are the most frequent.
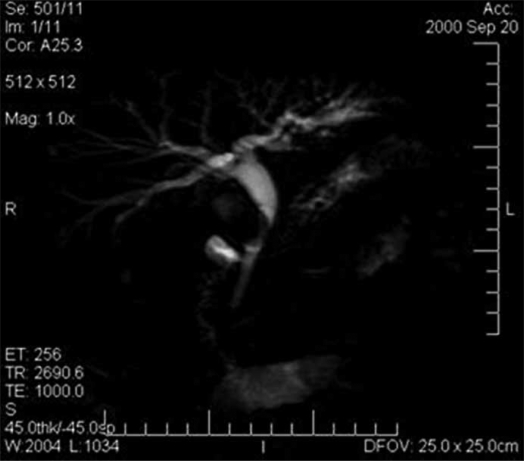
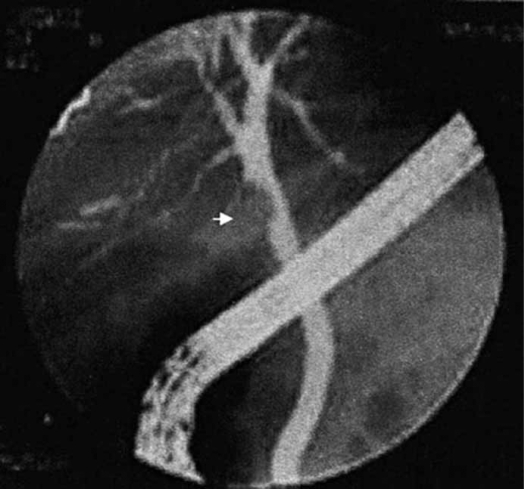
1) Mirizzi syndrome. – Mirizzi syndrome is defined as a common hepatic duct obstruction caused by an impacted stone in the gallbladder neck or cystic duct. Although its incidence is very low, at about 0.7–2.5% 38,44,45, this diagnosis requires US and magnetic resonance imaging (MRI) to reveal the presence of a large stone. Local inflammatory reaction at the site of stone intrusion can be diagnosed by CT. ERCP, PTC and especially MRCP can show the extrinsic narrowing that bows the main bile duct to the left, with a stricture usually long and smooth (Figure 1) 46,47,48.
Figure 1. .
MRCP of mirizzi syndrome.
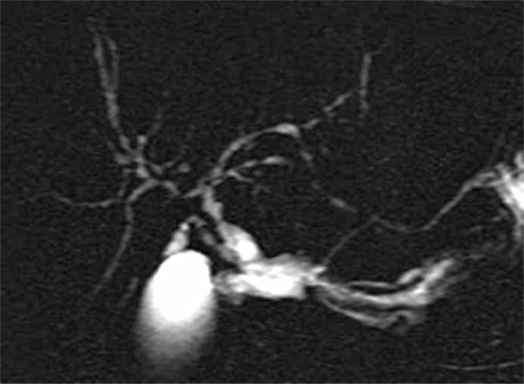
2) Primary sclerosing cholangitis (PSC) (Figure 2). – This autoimmune disorder affects periductal tissues of the biliary tree, leading to multifocal strictures of intrahepatic and extrahepatic bile duct. It is associated with ulcerative rectocolitis in up to 75% of cases. This pre-cancerous lesion can degenerate in 8% of cases 49. Management of a patient with PSC and suspected CC is difficult. In a patient with PSC, there are roughly two difficult situations. The first is the presence of localized bile duct stricture mimicking CC. In this setting, the patient should be extensively explored for indications in favour of PSC, including: (a) long-standing non-icteric cholestasis; (b) presence of ulcerative colitis; (c) radiologic features in intrahepatic bile ducts of distant strictures; and (d) liver biopsy showing aspects of PSC 50. The second difficult situation is a high suspicion of CC in a patient with PSC, because cholangiocarcinoma in the setting of PSC is of poor prognosis, and usually contra-indicates liver transplantation (LT). The presence of ECC should be highly suspected when the patient has had major changes in clinical symptoms, including during onset of the disease. However, results of LT in selected patients with PSC associated with early CC can be favourable providing the tumour is not associated with lymph node involvement and also providing the transplant procedure is initiated by radio-chemotherapy 51. CA19-9 is useful in this case. A value >100 U/ml has great sensibility and specificity for the diagnosis of malignant transformation 52,53. In this situation, histological or cytological examination of the stricture by means of biopsy or brush cytology is required 54.
Figure 2. .
MRCP of primary sclerosing cholangitis.
3) Secondary sclerosing cholangitis (SSC). – SSC is a disease that is morphologically similar to PSC but differs in pathological process. Among several infections that can lead to SSC and mimic ECC are:
3-1) Inflammatory pseudotumour (IPT). IPT is an entity that re-groups non-malignant lesions of the extrahepatic bile duct with inflammatory components. Histopathology findings are non-specific inflammation, fibrosis, cholangitis and granulomatosis 4,5,6,7,8,9,10,11,12,13. Aetiology remains unknown and the exact incidence of this disease is difficult to evaluate. However, between 5% and 20% of bile duct strictures are IPT, which represents almost all the benign aetiologies 4,5,6,7,8,9,10,11,12,13. Associations with phlebitis, Crohn disease and sclerosing cholangitis have been described 55,56,57,58,59. In the case of mimicking tumours of common bile duct, IPT occurs at 50 years, i.e. 10 years younger than CC occurs 9. It develops near extrahepatic bile duct and gallbladder. CA19-9 can be normal or slightly elevated 38. Radiological findings cannot accurately distinguish benign from malignant strictures. Indeed, 30% to 75% of IPTs show tumoral syndrome on abdominal CT or MRCP even though vascular invasion and/or encasement has never been described in IPT 6,7,8,9,12,14. Moreover, at least one half of tumours seem to be maligns at laparotomy 6,7,14. That's why Hadjis first called them “malignant masquerade” 8.
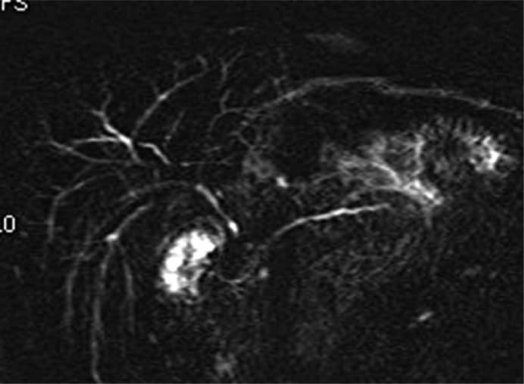
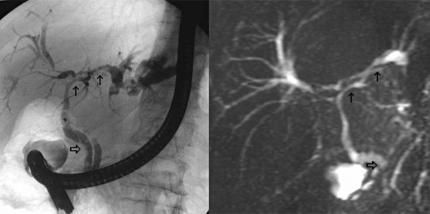
3-2) Autoimmune pancreatocholangitis (lymphoplasmacytic pancreatitis with cholangitis) (Figure 3). – Autoimmune pancreatocholangitis (AIP) is characterized by lymphoplasmacytic cellular infiltrates that may cause sclerosing inflammation of the biliary tree or pancreatic duct 60,61,62. Patients present with obstructive jaundice. Imaging shows an inflammatory mass of the lower part of the bile duct and the pancreas, with enlargement of the gland 38,63. The strictures may be long and multiple and often mimic PSC 62. Serum IgG4 value >100 mg/ml is helpful in distinguishing AIP from malignancy 61. In suspected cases, initial treatment with corticosteroid can be proposed 64.
Figure 3. .
MRCP of autoimmune pancreatocholangitis.
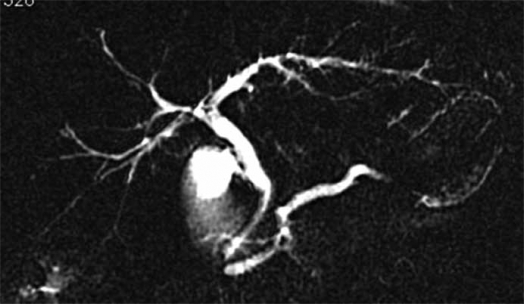
3-3) AIDS cholangiopathy (Figure 4). – First described by Margulis in 1986, this event has become rare since the introduction of antiviral therapy 65. Patients are generally in advanced stages of their disease 66. At MRCP or cholangiography, the entire biliary tree can be affected, but papillary stenosis with or without dilatation of the main pancreatic duct is unique in AIDS cholangiopathy and establishes the diagnosis 66.
Figure 4. .
MRCP of AIDS cholangiopathy
3-4) Other cholangitis. – Ischaemic cholangitis, recurrent pyogenic cholangitis and mast cell cholangiopathy can result in biochemical and radiological findings such as PSC 66. However, these aetiologies are rare, and clinical presentation often suggests non-malignant disease.
4) Portal biliopathy (Figure 5). – Patients presenting with bile duct stricture and portal cavernoma are usually suspected of having malignant disease. However, cavernoma can cause biliary obstruction. The mechanism is either compression by venous dilatation or ischaemia by cavernoma thrombosis 67,68,69. Extrahepatic portal vein thrombosis is the most common cause of portal biliopathy, but cirrhosis, portal vein fibrosis without cirrhosis and congenital hepatic fibrosis have been reported, too 70. Most patients have no symptoms 71,72,73. Direct cholangiographic findings, including segmental upstream dilatation, calibre irregularity, stricture and extrinsic impression on the bile duct due to collaterals, have been called “pseudocholangiocarcinoma signs” 71. However, transabdominal sonography or endoscopic sonography can reveal venous collaterals within or surrounding the extrahepatic bile duct 74. Portal MR and MRCP show paracholedochal and/or epicholedochal dilatations, and identify portosystemic shunts and morphology of the bile duct 69,71. Treatment by portosystemic shunt surgery allows regression of biliopathy only when the mechanism is compression 69.
Figure 5. .
CPRE and MRCP of portal biliopathy.
5) Adenoma and papilloma of the bile duct (Figure 6). – This is a rare benign epithelial tumour, with only 100 cases reported in the literature 75. The mean age of diagnosis of adenoma of the bile duct is 58 years, with a slight female predominance 76. The radiographic features are often difficult to distinguish from cholangiocarcinoma, particularly in the intraductal growing type 77. Most of these lesions predominate in common bile duct, especially in distal common duct and ampulla of Vater 78,79,80. US indicates non-shadowing intraluminal mass, sometimes with a pedicle 81. Endoscopic sonography of the bile ducts and cholangiography show complete or incomplete obstruction of the bile ducts by an endoluminal mass 80. Treatment of this lesion is surgery. Diagnostic may be suspected intraoperatively if the tumour appears polypoid and moveable within the bile duct. Then simple local resection is sufficient.
Figure 6. .
ERCP of bile duct adenoma.
Finally, ECC is difficult to differentiate from other aetiologies (benign or malignant). These findings suggest aggressive surgical therapy in front of suspected malignant stricture of the bile duct. Biopsy is not mandatory because of lack of sensibility and cost-effectiveness. However, we believe that a good knowledge of the literature and accurate semiological analysis can lead to diagnosis in most cases. In our experience, this approach has resulted in a dramatic decrease of mimicking tumours less than 10% (personal data). We believe that the dogma “accurate differentiation of benign and malignant hilar lesions is currently not possible outside the operating room, so resection remains the most reliable way to rule out biliary malignancy” has to be considered with great care and circumspection 9.
Intrahepatic cholangiocarcinoma (ICC)
ICC is a biliary tumour developed within the liver. The incidence is tending to rise throughout the world and, in all age groups, both genders, median size as well as tumour stage remain unchanged, suggesting a real increase rather than improvement in detection rate 3. Risk factors of ICC are PSC, hepatolithiasis, parasitic infections, chemical carcinogen exposure and viral hepatitis 3. Symptoms as well as biochemical investigation are often non-specific 1. Curative treatment consists in partial liver resection with lymph node dissection. Factors of poor prognosis are infiltrative type of ICC with satellite nodules and positive lymph nodes.
ICC develops in normal liver. Classically, in the mass-forming type it appears as a hypovascular tumour with fibrotic component that can induce portal compression 82. Capsular retraction and localized dilatation of peritumoral bile ducts are frequent. ICC is often associated with lymph node metastases.
ICC can be confused with fibrous metastases of carcinomas such as breast cancer and colorectal cancer 82. These tumours develop within normal liver like ICC, with similar age of incidence. Absence of the possible primary site, a relatively large tumour size and ancillary findings such as bile duct dilatation can be clues in differentiating mass-forming cholangiocarcinomas from metastases. If not, we advocate two approaches: 1) Biopsy of the lesion has not yet been performed: clinical examination, colonoscopy and mammography are useful, first to eliminate a primary related to liver metastases, and second because patients of 50 years of age or more are usually candidates for screening for these two diseases 83. If these examinations are negative, we retain the diagnosis of ICC and perform liver resection with lymph node dissection. 2) Biopsy has been realized but is non-conclusive: the lesion is a metastasis of adenocarcinoma from unknown primary (ACUP). Thirty per cent of metastases from ACUP are within the liver 84. Prognosis is very poor, with a median survival between 6 and 12 months 85,86,87. Even chemotherapy cannot improve survival significantly 85,87,88. Surgery has never been evaluated in ACUP because of often advanced disease with metastases at other sites. However, when resections have been made, no further study has found a poorer prognosis than chemotherapy 87,89,90. Therefore, in the case of a unique lesion with no extrahepatic disease, we advocate local resection with lymph node dissection because: (a) survival is not different from chemotherapy 89, (b) postoperative morbidity is low in liver resections, (c) histological and molecular analysis of the entire lesion can lead to the final diagnosis and permits accurate adjuvant therapy 91, and (d) in case atypical ICC treatment is complete.
In our opinion, ACUP has become a rare event. The incidence of ICC has been increasing for several decades, whereas the incidence of ACUP has been decreasing simultaneously. Some authors have postulated that a rise in incidence rate of ICC is a true increase because there are no significant changes in the staging and size of tumour at diagnosis 2. However, the reason for this increase remains unknown 92. We therefore believe that many ACUPs in past decades were ICC misdiagnosed. The rise in incidence of ICC is in fact due to improvement of diagnostic tools that help towards the correct diagnosis in most cases.
For this reason, all intrahepatic fibrous tumours with no evidence of primary tumour site must be considered as ICC. Biopsy is not mandatory, and surgical therapy with segmental liver resection and lymph node dissection must be performed.
References
- 1.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 4.Are C, Gonen M, D'Angelica M, DeMatteo RP, Fong Y, Blumgart LH, et al. Differential diagnosis of proximal biliary obstruction. Surgery. 2006;140:756–63. doi: 10.1016/j.surg.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Binkley CE, Eckhauser FE, Colletti LM. Unusual causes of benign biliary strictures with cholangiographic features of cholangiocarcinoma. J Gastrointest Surg. 2002;6:676–81. doi: 10.1016/s1091-255x(01)00062-2. [DOI] [PubMed] [Google Scholar]
- 6.Corvera CU, Blumgart LH, Darvishian F, Klimstra DS, DeMatteo R, Fong Y, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201:862–9. doi: 10.1016/j.jamcollsurg.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg. 2001;88:48–51. doi: 10.1046/j.1365-2168.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 8.Hadjis NS, Collier NA, Blumgart LH. Malignant masquerade at the hilum of the liver. Br J Surg. 1985;72:659–61. doi: 10.1002/bjs.1800720826. [DOI] [PubMed] [Google Scholar]
- 9.Koea J, Holden A, Chau K, McCall J. Differential diagnosis of stenosing lesions at the hepatic hilus. World J Surg. 2004;28:466–70. doi: 10.1007/s00268-004-7034-z. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama A, Imamura H, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Proximal bile duct stricture disguised as malignant neoplasm. Surgery. 1999;125:514–21. [PubMed] [Google Scholar]
- 11.Standfield NJ, Salisbury JR, Howard ER. Benign non-traumatic inflammatory strictures of the extrahepatic biliary system. Br J Surg. 1989;76:849–52. doi: 10.1002/bjs.1800760829. [DOI] [PubMed] [Google Scholar]
- 12.Verbeek PC, van Leeuwen DJ, de Wit LT, Reeders JW, Smits NJ, Bosma A, et al. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery. 1992;112:866–71. [PubMed] [Google Scholar]
- 13.Wetter LA, Ring EJ, Pellegrini CA, Way LW. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg 1991;161:57–62; discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 14.Knoefel WT, Prenzel KL, Peiper M, Hosch SB, Gundlach M, Eisenberger CF, et al. Klatskin tumors and Klatskin mimicking lesions of the biliary tree. Eur J Surg Oncol. 2003;29:658–61. doi: 10.1016/s0748-7983(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 15.Warshaw AL. Implications of peritoneal cytology for staging of early pancreatic cancer. Am J Surg 1991;161:26–9; discussion 29–30. [DOI] [PubMed] [Google Scholar]
- 16.de Bellis M, Sherman S, Fogel EL, Cramer H, Chappo J, McHenry L, Jr, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 2) Gastrointest Endosc. 2002;56:720–30. doi: 10.1067/mge.2002.129219. [DOI] [PubMed] [Google Scholar]
- 17.Foutch PG, Kerr DM, Harlan JR, Kummet TD. A prospective, controlled analysis of endoscopic cytotechniques for diagnosis of malignant biliary strictures. Am J Gastroenterol. 1991;86:577–80. [PubMed] [Google Scholar]
- 18.Glasbrenner B, Ardan M, Boeck W, Preclik G, Moller P, Adler G. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy. 1999;31:712–17. doi: 10.1055/s-1999-73. [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC, Baron TH, Stadheim LM, Kipp BR, Sebo TJ, Salomao DR. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol. 2004;99:1464–9. doi: 10.1111/j.1572-0241.2004.30845.x. [DOI] [PubMed] [Google Scholar]
- 20.Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG, et al. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383–90. doi: 10.1016/s0016-5107(00)70435-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee JG, Leung JW, Baillie J, Layfield LJ, Cotton PB. Benign, dysplastic, or malignant-making sense of endoscopic bile duct brush cytology: results in 149 consecutive patients. Am J Gastroenterol. 1995;90:722–6. [PubMed] [Google Scholar]
- 22.Macken E, Drijkoningen M, Van Aken E, Van Steenbergen W. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–9. [PubMed] [Google Scholar]
- 23.Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–7. doi: 10.1136/gut.40.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–72. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 25.Pugliese V, Conio M, Nicolo G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520–6. doi: 10.1016/s0016-5107(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 26.Stewart CJ, Mills PR, Carter R, O'Donohue J, Fullarton G, Imrie CW, et al. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54:449–55. doi: 10.1136/jcp.54.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logrono R, Kurtycz DF, Molina CP, Trivedi VA, Wong JY, Block KP. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at two university hospitals. Arch Pathol Lab Med. 2000;124:387–92. doi: 10.5858/2000-124-0387-AOFNDO. [DOI] [PubMed] [Google Scholar]
- 28.Mohandas KM, Swaroop VS, Gullar SU, Dave UR, Jagannath P, DeSouza LJ. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointest Endosc. 1994;40:150–4. doi: 10.1016/s0016-5107(94)70157-1. [DOI] [PubMed] [Google Scholar]
- 29.Parasher VK, Huibregtse K. Endoscopic retrograde wire-guided cytology of malignant biliary strictures using a novel scraping brush. Gastrointest Endosc. 1998;48:288–90. doi: 10.1016/s0016-5107(98)70193-2. [DOI] [PubMed] [Google Scholar]
- 30.de Bellis M, Fogel EL, Sherman S, Watkins JL, Chappo J, Younger C, et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003;58:176–82. doi: 10.1067/mge.2003.345. [DOI] [PubMed] [Google Scholar]
- 31.Howell DA, Parsons WG, Jones MA, Bosco JJ, Hanson BL. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc. 1996;43:498–502. doi: 10.1016/s0016-5107(96)70294-8. [DOI] [PubMed] [Google Scholar]
- 32.Wiersema MJ, Hawes RH, Tao LC, Wiersema LM, Kopecky KK, Rex DK, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–9. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 33.Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–8. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 34.Fogel EL, deBellis M, McHenry L, Watkins JL, Chappo J, Cramer H, et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006;63:71–7. doi: 10.1016/j.gie.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 35.Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–13. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 36.Fritscher-Ravens A, Broering DC, Sriram PV, Topalidis T, Jaeckle S, Thonke F, et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc. 2000;52:534–40. doi: 10.1067/mge.2000.109589. [DOI] [PubMed] [Google Scholar]
- 37.Rosch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390–6. doi: 10.1016/s0016-5107(04)01732-8. [DOI] [PubMed] [Google Scholar]
- 38.Blumgart LH. Surgery of the liver, biliary tract, and pancreas. 2006. [Google Scholar]
- 39.Chijiiwa K, Kimura H, Tanaka M. Malignant potential of the gallbladder in patients with anomalous pancreaticobiliary ductal junction. The difference in risk between patients with and without choledochal cyst. Int Surg. 1995;80:61–4. [PubMed] [Google Scholar]
- 40.Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–15. doi: 10.1245/aso.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Warshaw AL, Welch JP. Extrahepatic biliary obstruction by metastatic colon carcinoma. Ann Surg. 1978;188:593–7. doi: 10.1097/00000658-197811000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takasan H, Kitamura O, Ozawa K, Honjo I. Metastatic cancer of the liver resulting in obstruction of the extrahepatic bile duct. Am J Surg. 1973;125:782–5. doi: 10.1016/0002-9610(73)90187-6. [DOI] [PubMed] [Google Scholar]
- 43.Maroy B, Moullot P, Daloubeix H, Lavaud H, Lancret C. Intraductal biliary metastasis of a colonic cancer. Ann Radiol. 1988;31:309–11. [PubMed] [Google Scholar]
- 44.Bower TC, Nagorney DM. Mirizzi syndrome. HPB Surg 1988;1:67–74; discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt DR. Another paper on Mirizzi Syndrome? ANZ J Surg. 2004;74:826. doi: 10.1111/j.1445-1433.2004.03242.x. [DOI] [PubMed] [Google Scholar]
- 46.Becker CD, Grossholz M, Mentha G, de Peyer R, Terrier F. MR cholangiopancreatography: technique, potential indications, and diagnostic features of benign, postoperative, and malignant conditions. Eur Radiol. 1997;7:865–74. doi: 10.1007/s003300050220. [DOI] [PubMed] [Google Scholar]
- 47.Fulcher AS, Turner MA, Capps GW. MR cholangiography: technical advances and clinical applications. Radiographics 1999;19:25–41; discussion 41–4. [DOI] [PubMed] [Google Scholar]
- 48.Soto JA, Yucel EK, Barish MA, Chuttani R, Ferrucci JT. MR cholangiopancreatography after unsuccessful or incomplete ERCP. Radiology. 1996;199:91–8. doi: 10.1148/radiology.199.1.8633178. [DOI] [PubMed] [Google Scholar]
- 49.Broome U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–15. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaRusso NF, Shneider BL, Black D, Gores GJ, James SP, Doo E, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–64. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 51.Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451–8; discussion 458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–40. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 53.Nichols JC, Gores GJ, LaRusso NF, Wiesner RH, Nagorney DM, Ritts RE., Jr Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874–9. doi: 10.1016/s0025-6196(12)60696-x. [DOI] [PubMed] [Google Scholar]
- 54.Furmanczyk PS, Grieco VS, Agoff SN. Biliary brush cytology and the detection of cholangiocarcinoma in primary sclerosing cholangitis: evaluation of specific cytomorphologic features and CA19-9 levels. Am J Clin Pathol. 2005;124:355–60. doi: 10.1309/J030-JYPW-KQTH-CLNJ. [DOI] [PubMed] [Google Scholar]
- 55.Horiuchi R, Uchida T, Kojima T, Shikata T. Inflammatory pseudotumor of the liver. Clinicopathologic study and review of the literature. Cancer. 1990;65:1583–90. doi: 10.1002/1097-0142(19900401)65:7<1583::aid-cncr2820650722>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Amankonah TD, Strom CB, Vierling JM, Petrovic LM. Inflammatory pseudotumor of the liver as the first manifestation of Crohn's disease. Am J Gastroenterol. 2001;96:2520–2. doi: 10.1111/j.1572-0241.2001.04079.x. [DOI] [PubMed] [Google Scholar]
- 57.Jafri SZ, Bree RL, Agha FP, Schwab RE. Inflammatory pseudotumor from sclerosing cholangitis. J Comput Assist Tomogr. 1983;7:902–4. doi: 10.1097/00004728-198310000-00031. [DOI] [PubMed] [Google Scholar]
- 58.Nakanuma Y, Tsuneyama K, Masuda S, Tomioka T. Hepatic inflammatory pseudotumor associated with chronic cholangitis: report of three cases. Hum Pathol. 1994;25:86–91. doi: 10.1016/0046-8177(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 59.Nonomura A, Minato H, Shimizu K, Kadoya M, Matsui O. Hepatic hilar inflammatory pseudotumor mimicking cholangiocarcinoma with cholangitis and phlebitis – a variant of primary sclerosing cholangitis? Pathol Res Pract 1997;193:519–25; discussion 526. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi K, Nakazawa T, Ohara H, Ando T, Takada H, Tanaka H, et al. Autoimmune sclerosing cholangiopancreatitis with little pancreatic involvements by imaging findings. Hepatogastroenterology. 2007;54:2146–51. [PubMed] [Google Scholar]
- 61.Hochwald SN, Hemming AW, Draganov P, Vogel SB, Dixon LR, Grobmyer SR. Elevation of serum IgG4 in Western patients with autoimmune sclerosing pancreatocholangitis: a word of caution. Ann Surg Oncol 2008. [DOI] [PubMed] [Google Scholar]
- 62.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–95. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 63.Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics. 2008;28:157–70. doi: 10.1148/rg.281065188. [DOI] [PubMed] [Google Scholar]
- 64.Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreato-cholangitis responsive to steroid therapy. Lancet. 1999;354:43–4. doi: 10.1016/s0140-6736(99)00603-0. [DOI] [PubMed] [Google Scholar]
- 65.Margulis SJ, Honig CL, Soave R, Govoni AF, Mouradian JA, Jacobson IM. Biliary tract obstruction in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:207–10. doi: 10.7326/0003-4819-105-2-207. [DOI] [PubMed] [Google Scholar]
- 66.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063–74. doi: 10.1002/hep.21405. [DOI] [PubMed] [Google Scholar]
- 67.Akaki S, Kobayashi H, Sasai N, Tsunoda M, Kuroda M, Kanazawa S, et al. Bile duct stenosis due to portal cavernomas: MR portography and MR cholangiopancreatography demonstration. Abdom Imaging. 2002;27:58–60. doi: 10.1007/s00261-001-0038-3. [DOI] [PubMed] [Google Scholar]
- 68.Chandra R, Kapoor D, Tharakan A, Chaudhary A, Sarin SK. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086–92. doi: 10.1046/j.1440-1746.2001.02562.x. [DOI] [PubMed] [Google Scholar]
- 69.Dhiman RK, Puri P, Chawla Y, Minz M, Bapuraj JR, Gupta S, et al. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–52. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 70.Sarin SK, Bhatia V, Makwana U. Portal biliopathy in extrahepatic portal venous obstruction. Indian J Gastroenterol. 1992;11 Suppl 1:A82. [Google Scholar]
- 71.Shin SM, Kim S, Lee JW, Kim CW, Lee TH, Lee SH, et al. Biliary abnormalities associated with portal biliopathy: evaluation on MR cholangiography. Am J Roentgenol. 2007;188:W341–7. doi: 10.2214/AJR.05.1649. [DOI] [PubMed] [Google Scholar]
- 72.Khuroo MS, Yattoo GN, Zargar SA, Javid G, Dar MY, Khan BA, et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–13. [PubMed] [Google Scholar]
- 73.Gibson JB, Johnston GW, Fulton TT, Rodgers HW. Extrahepatic portal-venous obstruction. Br J Surg. 1965;52:129–39. doi: 10.1002/bjs.1800520211. [DOI] [PubMed] [Google Scholar]
- 74.Umphress JL, Pecha RE, Urayama S. Biliary stricture caused by portal biliopathy: diagnosis by EUS with Doppler US. Gastrointest Endosc. 2004;60:1021–4. doi: 10.1016/s0016-5107(04)02216-3. [DOI] [PubMed] [Google Scholar]
- 75.Kunisaki SM, Hertl M, Bodner BE, Cosimi AB. Mirizzi syndrome secondary to an adenoma of the cystic duct. J Hepatobil Pancreat Surg. 2005;12:159–62. doi: 10.1007/s00534-004-0970-z. [DOI] [PubMed] [Google Scholar]
- 76.Allaire GS, Rabin L, Ishak KG, Sesterhenn IA. Bile duct adenoma. A study of 152 cases. Am J Surg Pathol. 1988;12:708–15. doi: 10.1097/00000478-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Hasebe T, Sakamoto M, Mukai K, Kawano N, Konishi M, Ryu M, et al. Cholangiocarcinoma arising in bile duct adenoma with focal area of bile duct hamartoma. Virchows Arch. 1995;426:209–13. doi: 10.1007/BF00192644. [DOI] [PubMed] [Google Scholar]
- 78.Cattell RB, Braasch JW, Kahn F. Polypoid epithelial tumors of the bile ducts. N Engl J Med. 1962;266:57–61. doi: 10.1056/NEJM196201112660201. [DOI] [PubMed] [Google Scholar]
- 79.Dowdy GS, Jr, Olin WG, Jr, Shelton EL, Jr, Waldron GW. Benign tumors of the extrahepatic bile ducts. Report of three cases and review of the literature. Arch Surg. 1962;85:503–13. doi: 10.1001/archsurg.1962.01310030151024. [DOI] [PubMed] [Google Scholar]
- 80.Kawakatsu M, Vilgrain V, Zins M, Vullierme M, Belghiti J, Menu Y. Radiologic features of papillary adenoma and papillomatosis of the biliary tract. Abdom Imaging. 1997;22:87–90. doi: 10.1007/s002619900147. [DOI] [PubMed] [Google Scholar]
- 81.Buckley JG, Salimi Z. Villous adenoma of the common bile duct. Abdom Imaging. 1993;18:245–6. doi: 10.1007/BF00198114. [DOI] [PubMed] [Google Scholar]
- 82.Choi BI, Lee JM, Han JK. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging. 2004;29:548–57. doi: 10.1007/s00261-004-0188-1. [DOI] [PubMed] [Google Scholar]
- 83.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Supple 6:VI:1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mousseau M, Schaerer R, Lutz JM, Menegoz F, Faure H, Swiercz P. Hepatic metastasis of unknown primary site. Bull Cancer. 1991;78:725–36. [PubMed] [Google Scholar]
- 85.Pouessel D, Thezenas S, Culine S, Becht C, Senesse P, Ychou M. Hepatic metastases from carcinomas of unknown primary site. Gastroenterol Clin Biol. 2005;29:1224–32. doi: 10.1016/s0399-8320(05)82205-5. [DOI] [PubMed] [Google Scholar]
- 86.Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257–63. doi: 10.1056/NEJM199307223290407. [DOI] [PubMed] [Google Scholar]
- 87.Culine S, Kramar A, Saghatchian M, Bugat R, Lesimple T, Lortholary A, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20:4679–83. doi: 10.1200/JCO.2002.04.019. [DOI] [PubMed] [Google Scholar]
- 88.Sporn JR, Greenberg BR. Empirical chemotherapy for adenocarcinoma of unknown primary tumor site. Semin Oncol. 1993;20:261–7. [PubMed] [Google Scholar]
- 89.Verhoef C, Kuiken BW, JN IJ, de Wilt JH. Partial hepatic resection for liver metastases of non-colorectal origin, is it justified? Hepatogastroenterology. 2007;54:1517–21. [PubMed] [Google Scholar]
- 90.Hawksworth J, Geisinger K, Zagoria R, Kavanagh P, Howerton R, Levine EA, et al. Surgical and ablative treatment for metastatic adenocarcinoma to the liver from unknown primary tumor. Am Surg. 2004;70:512–7. [PubMed] [Google Scholar]
- 91.Schneider BJ, El-Rayes B, Muler JH, Philip PA, Kalemkerian GP, Griffith KA, et al. Phase II trial of carboplatin, gemcitabine, and capecitabine in patients with carcinoma of unknown primary site. Cancer. 2007;110:770–5. doi: 10.1002/cncr.22857. [DOI] [PubMed] [Google Scholar]
- 92.Blendis L, Halpern Z. An increasing incidence of cholangiocarcinoma: why? Gastroenterology. 2004;127:1008–9. doi: 10.1053/j.gastro.2004.07.035. [DOI] [PubMed] [Google Scholar]