Abstract
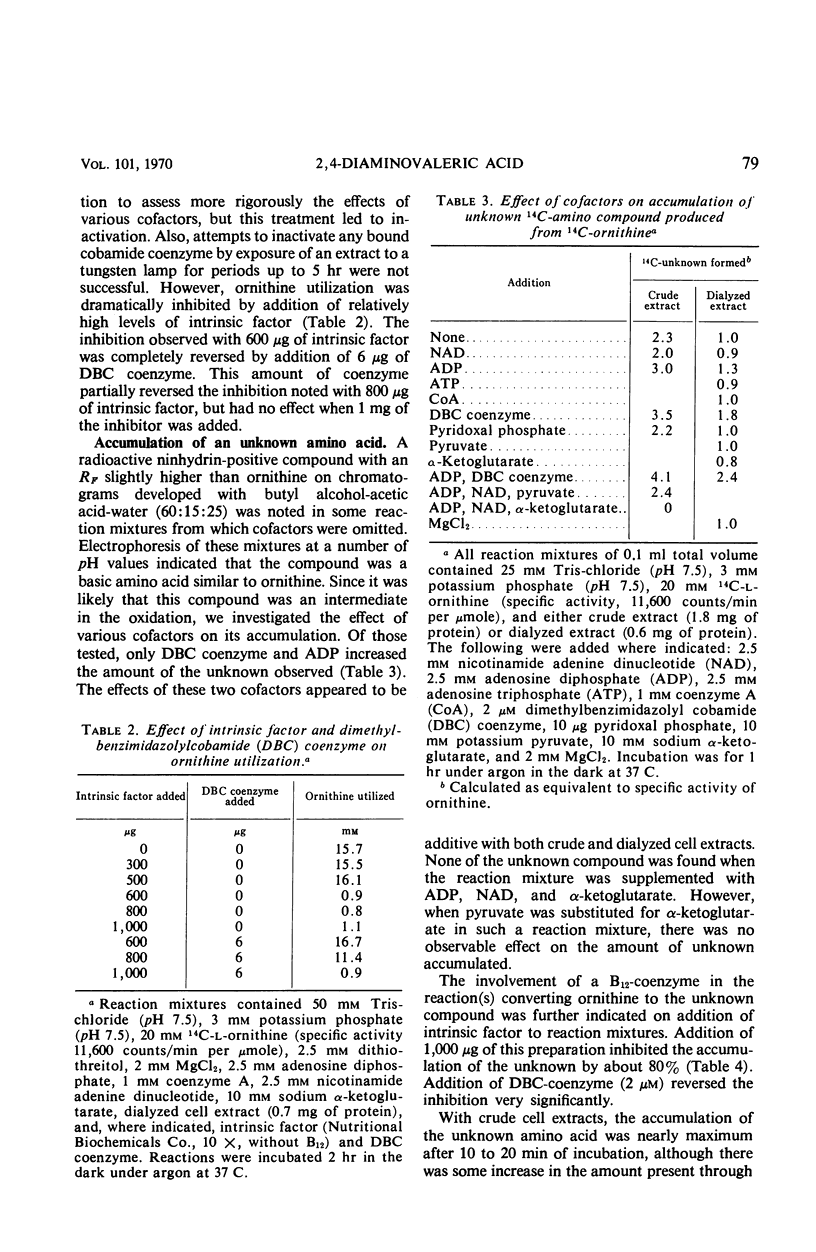
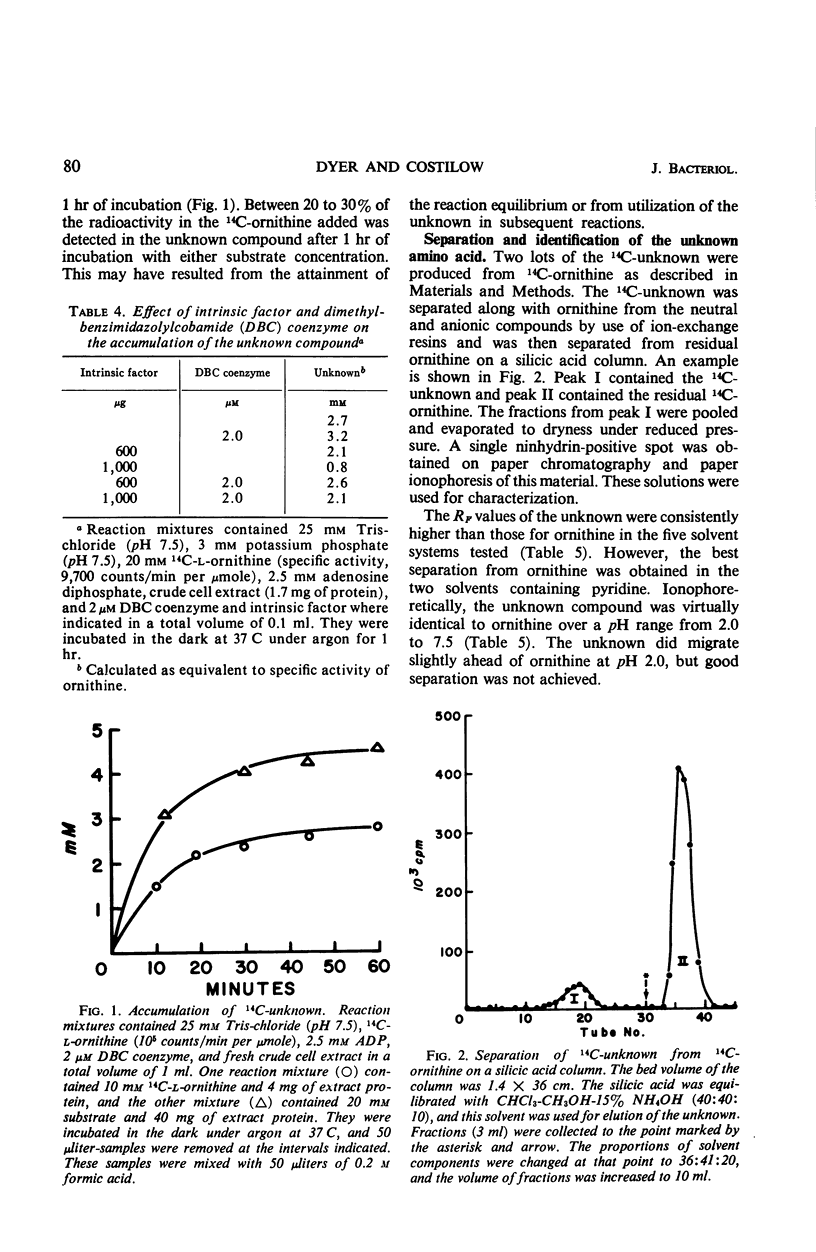
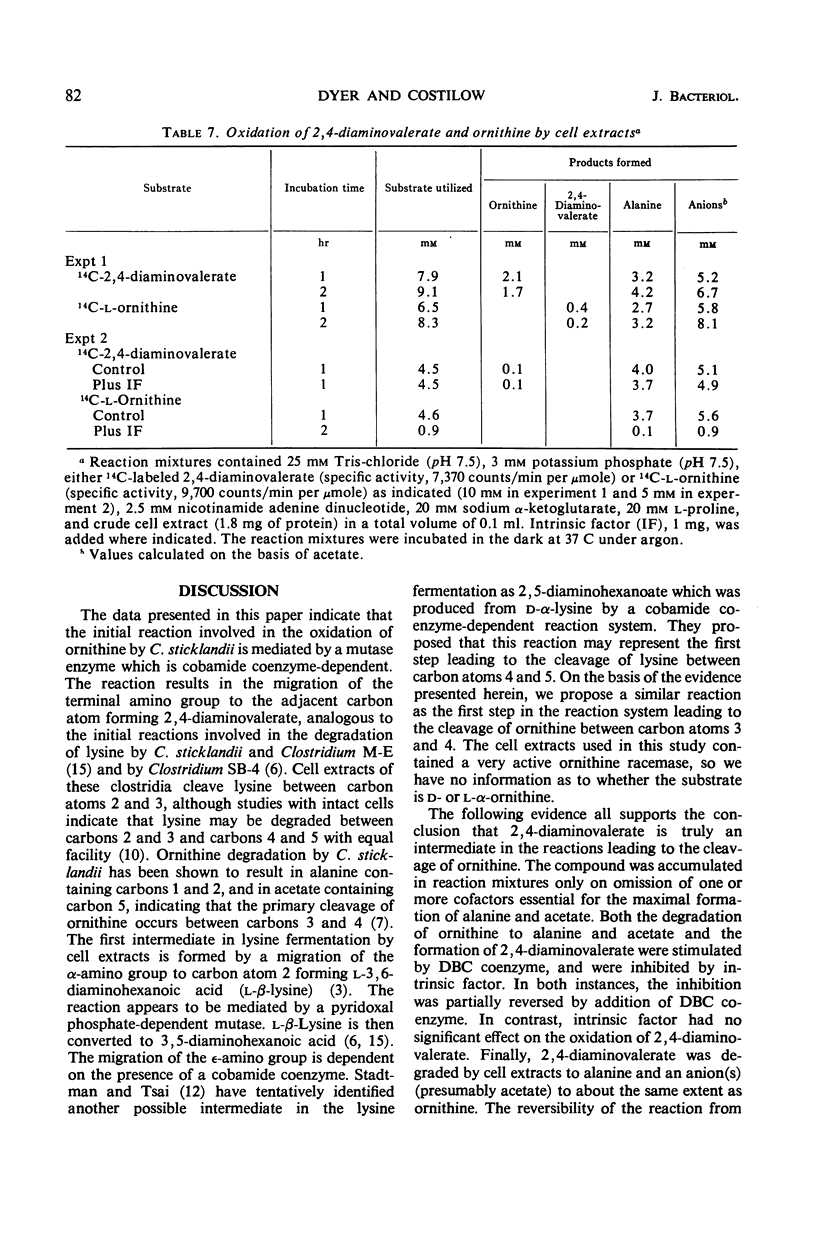
The oxidation of ornithine in the presence of proline by crude extracts of Clostridium sticklandii cells was stimulated by nicotinamide adenine dinucleotide, coenzyme A, α-ketoglutarate, dimethylbenzimidazolyl cobamide (DBC) coenzyme, MgCl2, and adenosine diphosphate. Deletion of various cofactors resulted in the accumulation of a new dibasic amino acid which was identified as 2,4-diaminovaleric acid. Both the oxidation of ornithine to alanine and acetate and the conversion of ornithine to 2,4-diaminovaleric acid were stimulated by addition of DBC coenzyme, and both were inhibited by intrinsic factor, an inhibitor of cobamide coenzyme-dependent reactions. This inhibition was reversed by addition of DBC coenzyme. However, the oxidation of 2,4-diaminovaleric acid was insensitive to added intrinsic factor. The data indicate that 2,4-diaminovaleric acid represents the first intermediate in the oxidation of ornithine by C. sticklandii.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costilow R. N., Laycock L. Proline as an intermediate in the reductive deamination of ornithine to delta-aminovaleric acid. J Bacteriol. 1968 Oct;96(4):1011–1020. doi: 10.1128/jb.96.4.1011-1020.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R. N., Rochovansky O. M., Barker H. A. Isolation and identification of beta-lysine as an intermediate in lysine fermentation. J Biol Chem. 1966 Apr 10;241(7):1573–1580. [PubMed] [Google Scholar]
- Dekker E. E., Barker H. A. Identification and cobamide coenzyme-dependent formation of 3,5-diaminohexanoic acid, an intermediate in lysine fermentation. J Biol Chem. 1968 Jun 25;243(12):3232–3237. [PubMed] [Google Scholar]
- Dyer J. K., Costilow R. N. Fermentation of ornithine by Clostridium sticklandii. J Bacteriol. 1968 Nov;96(5):1617–1622. doi: 10.1128/jb.96.5.1617-1622.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rimerman E. A., Barker H. A. Formation and identification of 3-keto-5-aminohexanoic acid, a probable intermediate in lysine fermentation. J Biol Chem. 1968 Dec 10;243(23):6151–6160. [PubMed] [Google Scholar]
- STADTMAN T. C. ANAEROBIC DEGRADATION OF LYSINE. II. COFACTOR REQUIREMENTS AND PROPERTIES OF THE SOLUBLE ENZYME SYSTEM. J Biol Chem. 1963 Aug;238:2766–2773. [PubMed] [Google Scholar]
- STADTMAN T. C., WHITE F. H., Jr Tracer studies on ornithine, lysine, and formate metabolism in an amino acid fermenting Clostridium. J Bacteriol. 1954 Jun;67(6):651–657. doi: 10.1128/jb.67.6.651-657.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Tsai L. A cobamide coenzyme dependent migration of the epsilon-amino group of D-lysine. Biochem Biophys Res Commun. 1967 Sep 27;28(6):920–926. doi: 10.1016/0006-291x(67)90067-8. [DOI] [PubMed] [Google Scholar]
- Tsai L., Stadtman T. C. Anaerobic degradation of lysine. IV. Cobamide coenzyme-dependent migration of an amino group from carbon 6 of beta-lysine (3,6-diaminohexanoate) to carbon 5 forming a new naturally occurring amino acid, 3,5-diaminohexanoate. Arch Biochem Biophys. 1968 Apr;125(1):210–225. doi: 10.1016/0003-9861(68)90656-5. [DOI] [PubMed] [Google Scholar]


