Abstract
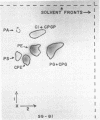
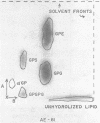
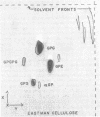
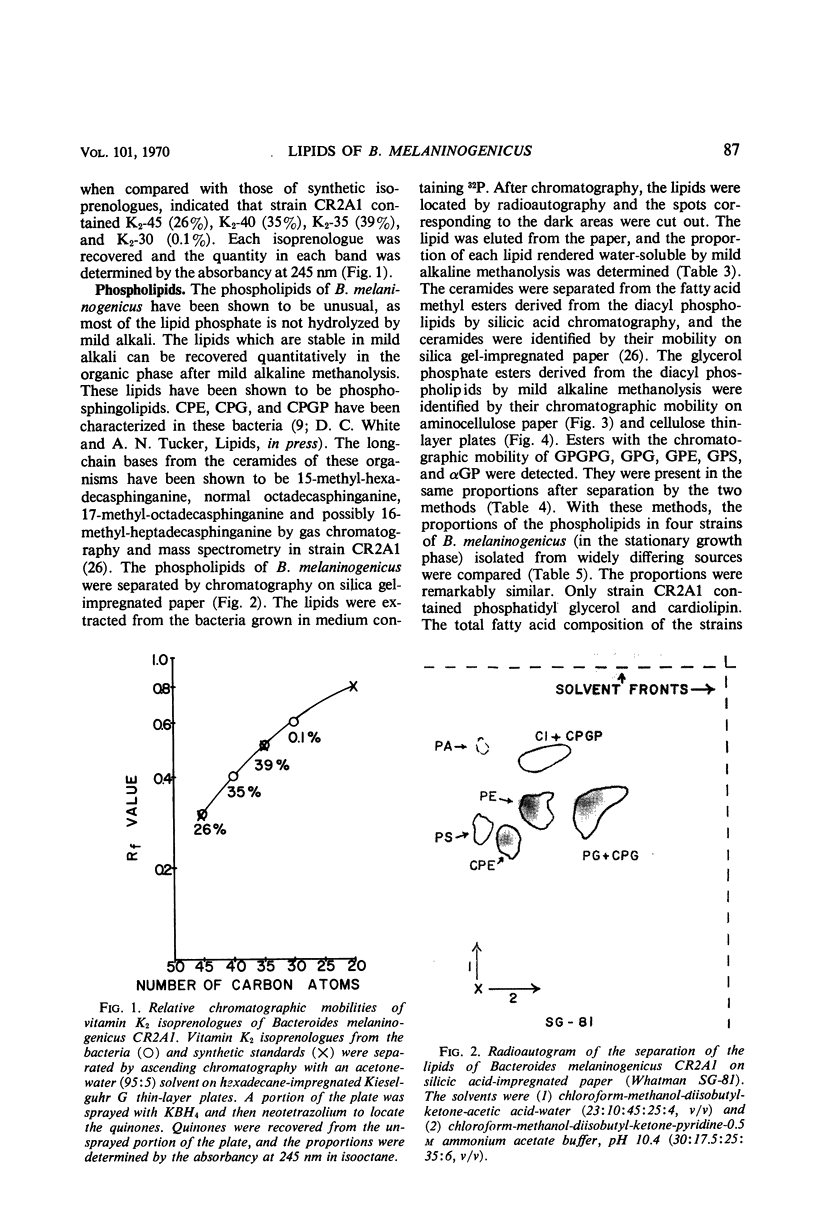
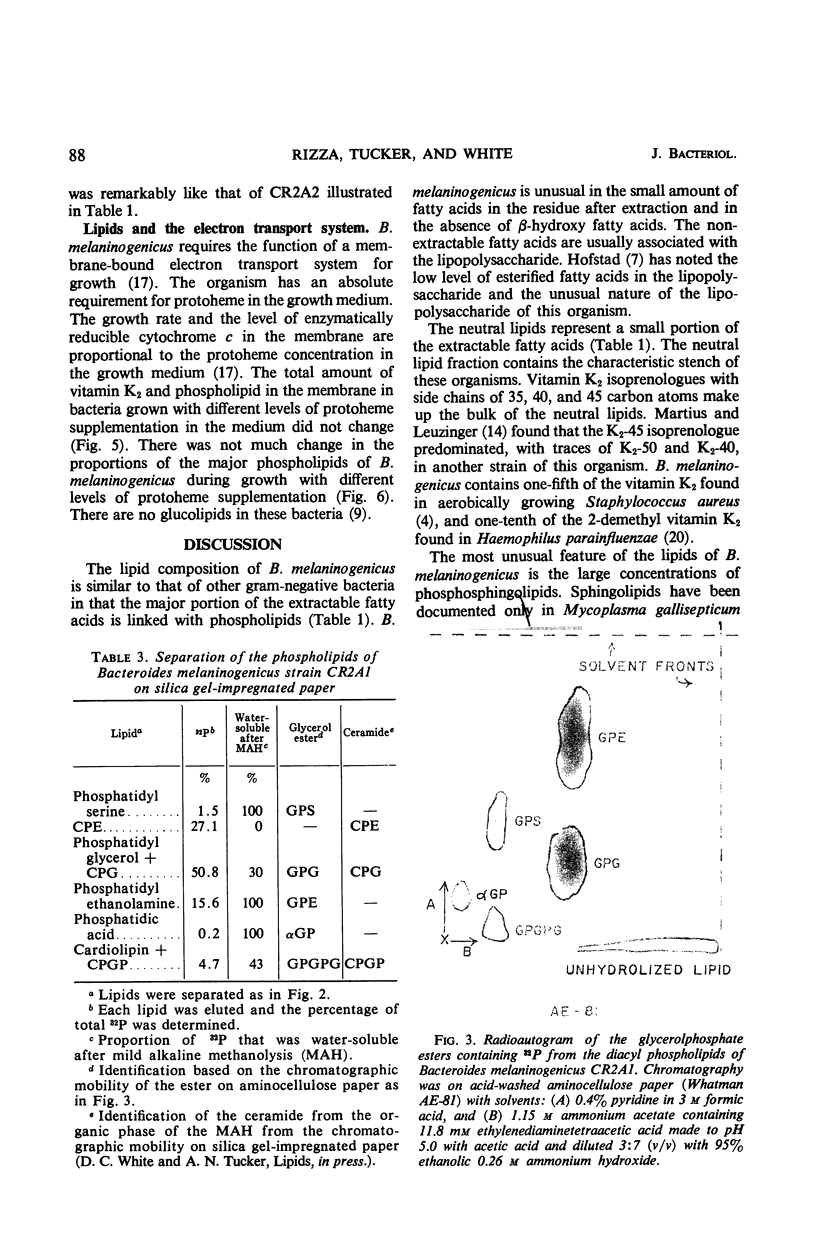
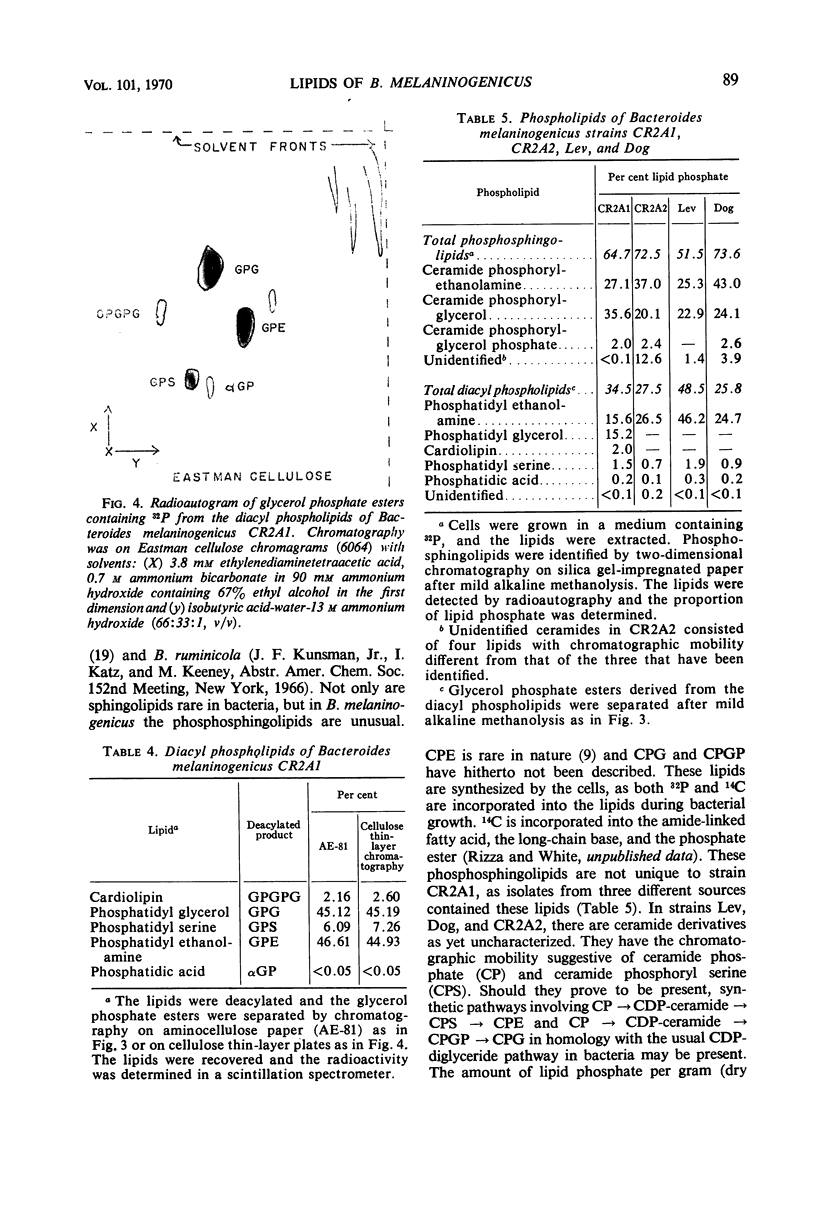
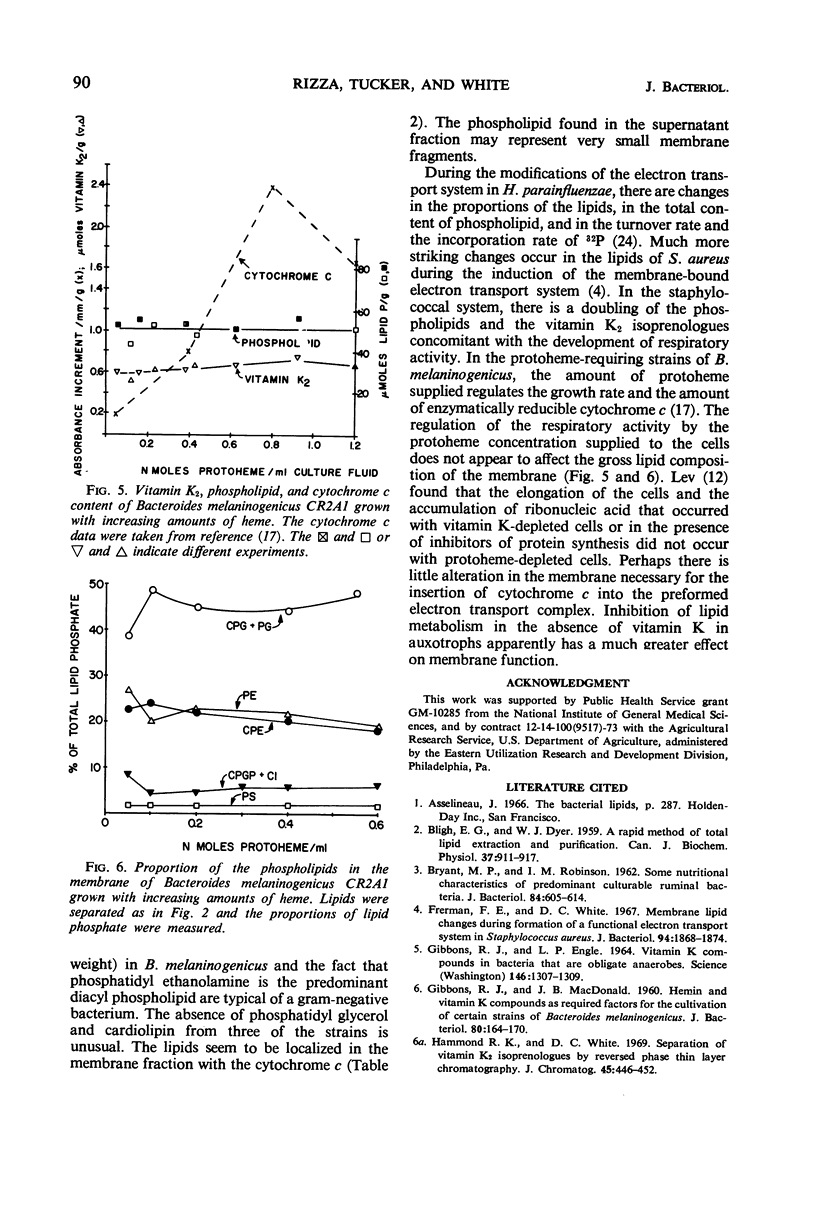
The lipids of Bacteroides melaninogenicus were readily extractable with chloroform-methanol. Three per cent of the fatty acids were not extractable. The neutral lipids contained 4% of the extractable fatty acids, the stench characteristic of these organisms, and 0.5 μmole of vitamin K2 isoprenologues K2-35, K2-40, and K2-45 per g (dry weight). This is one-fifth to one-tenth of the vitamin K2 level found in other bacteria. Ninety-six per cent of the extractable fatty acids were associated with the phospholipids (60 μmoles of lipid phosphate/g, dry weight), which consisted of the diacyl lipids phosphatidic acid, phosphatidyl serine, and phosphatidyl ethanolamine (with phosphatidyl glycerol and cardiolipin in one strain). The unusual phosphosphingolipids ceramide phosphorylethanolamine, ceramide phosphorylglycerol, and ceramide phosphorylglycerol phosphate accounted for 50 to 70% of the lipid phosphate. In protoheme-requiring strains, the protoheme concentration in the growth medium regulated the growth rate and the amount of enzymatically reducible cytochrome c. There were no gross changes in the lipid composition in cells containing different levels of enzymatically reducible cytochrome c.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., ENGLE L. P. VITAMIN K COMPOUNDS IN BACTERIA THAT ARE OBLIGATE ANAEROBES. Science. 1964 Dec 4;146(3649):1307–1309. doi: 10.1126/science.146.3649.1307. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Separation of vitamin K2 isoprenologues by reversed-phase thin-layer chromatography. J Chromatogr. 1969 Dec 23;45(3):446–452. doi: 10.1016/s0021-9673(01)86242-7. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Chemical characteristics of Bacteroides melaninogenicus endotoxin. Arch Oral Biol. 1968 Sep;13(9):1149–1155. doi: 10.1016/0003-9969(68)90067-8. [DOI] [PubMed] [Google Scholar]
- LEV M. Apparent requirement for vitamin K of rumen strains of Fusiformis nigrescens. Nature. 1958 Jan 18;181(4603):203–204. doi: 10.1038/181203a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBach J. P., White D. C. Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res. 1969 Sep;10(5):528–534. [PubMed] [Google Scholar]
- Lev M. Vitamin K deficiency in Fusiformis nigrescens. I. Influence on whole cells and cell envelope characteristics. J Bacteriol. 1968 Jun;95(6):2317–2324. doi: 10.1128/jb.95.6.2317-2324.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIUS C., LEUZINGER W. UBER DIE UMWANDLUNG VON K-VITAMINEN IN EINEM K-HETEROTROPHEN ANAEROBIER(FUSIFORMIS NIGRESCENS) Biochem Z. 1964 Aug 11;340:304–315. [PubMed] [Google Scholar]
- NESBITT J. A., 3rd, LENNARZ W. J. COMPARISON OF LIPIDS AND LIPOPOLYSACCHARIDE FROM THE BACILLARY AND L FORMS OF PROTEUS P18. J Bacteriol. 1965 Apr;89:1020–1025. doi: 10.1128/jb.89.4.1020-1025.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza V., Sinclair P. R., White D. C., Cuorant P. R. Electron transport system of the protoheme-requiring anaerobe Bacteroides melaninogenicus. J Bacteriol. 1968 Sep;96(3):665–671. doi: 10.1128/jb.96.3.665-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER S. J., MACDONALD J. B., GIBBONS R. J. Biochemical characteristics of Bacteroides melaninogenicus. A study of thirty-one strains. Arch Oral Biol. 1962 Nov-Dec;7:685–691. doi: 10.1016/0003-9969(62)90117-6. [DOI] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JENSEN R. G., GANDER G. W., MOROWITZ H. J. LIPID COMPOSITION AND SYNTHESIS IN THE PLEUROPNEUMONIA-LIKE ORGANISM MYCOPLASMA GALLISEPTICUM. J Bacteriol. 1963 Sep;86:370–379. doi: 10.1128/jb.86.3.370-379.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during changes in the proportions of membrane-bound respiratory pigments in Haemophilus parainfluenzae. J Bacteriol. 1969 Jan;97(1):199–209. doi: 10.1128/jb.97.1.199-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N., Sweeley C. C. Characterization of the iso-branched sphinganines from the ceramide phospholipids of Bacteroides melaninogenicus. Biochim Biophys Acta. 1969 Dec 17;187(4):527–532. doi: 10.1016/0005-2760(69)90050-2. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]