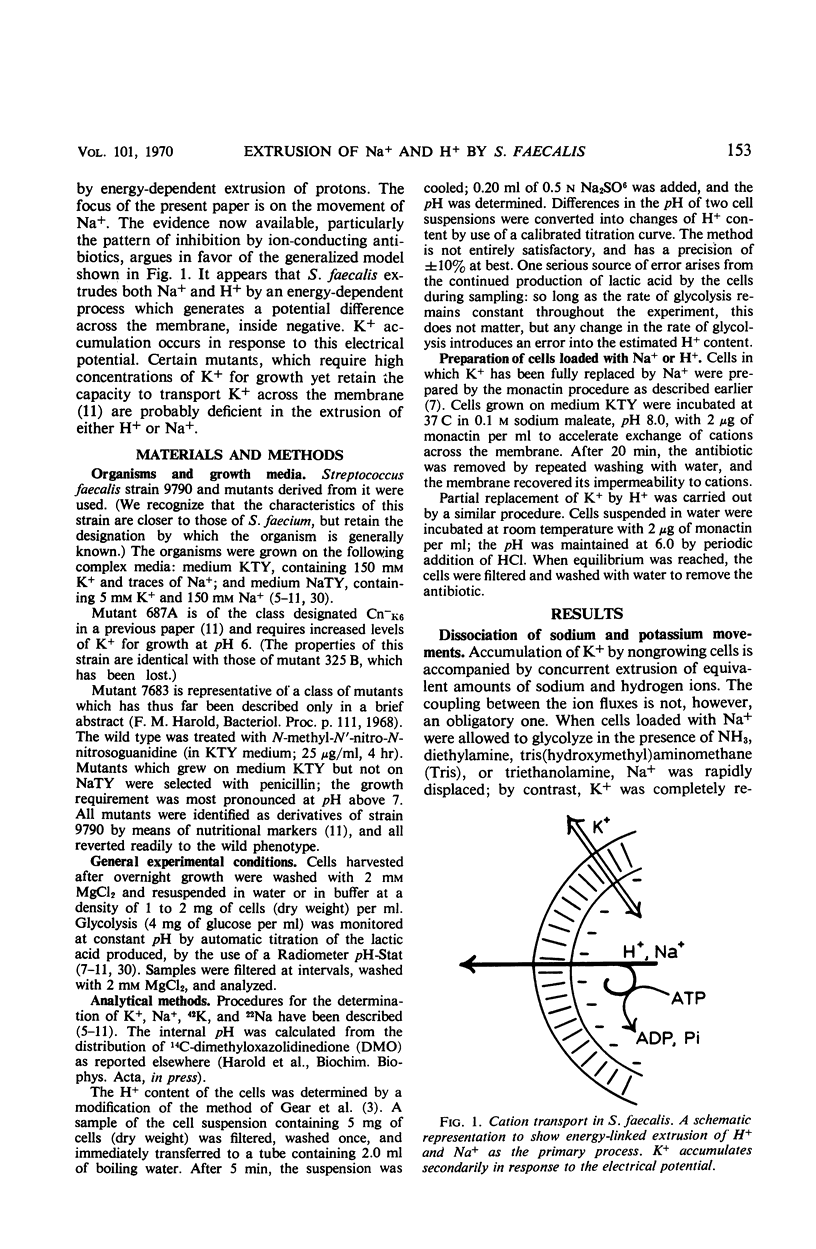
Abstract
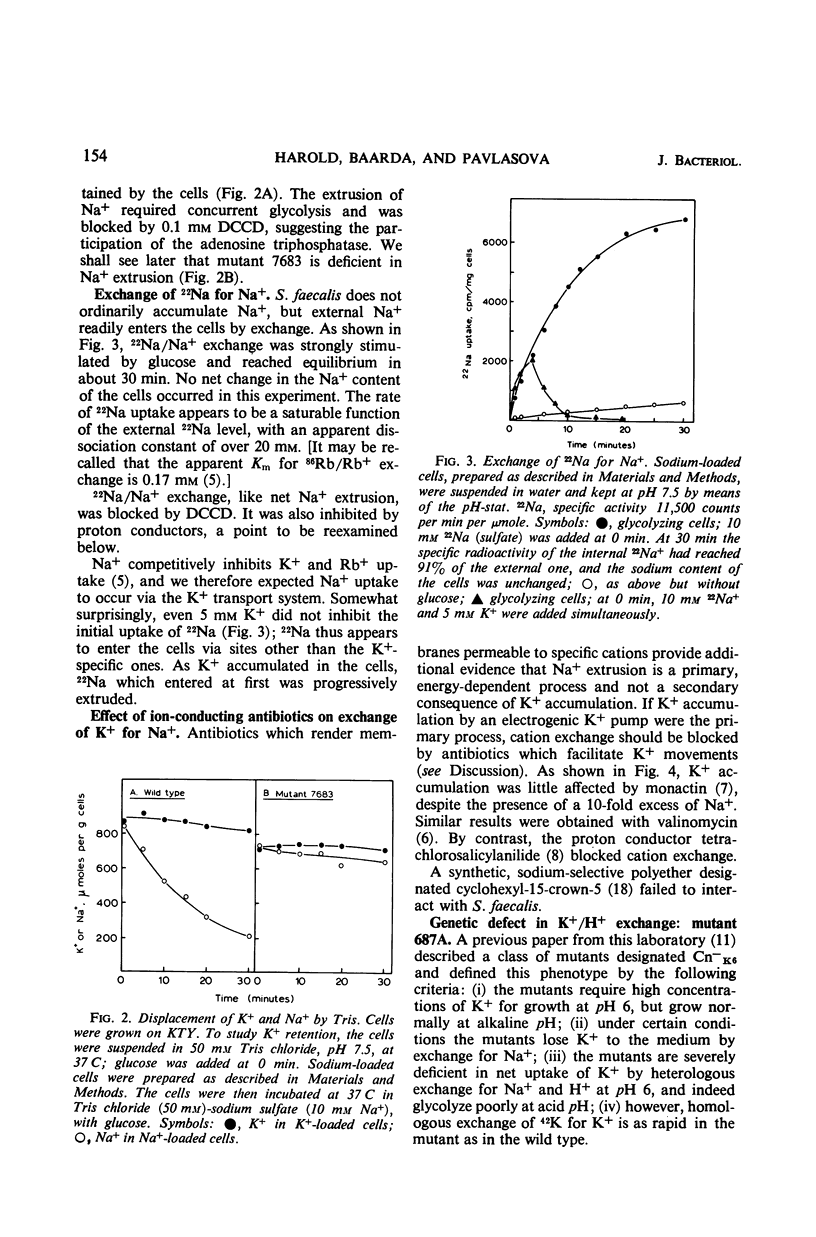
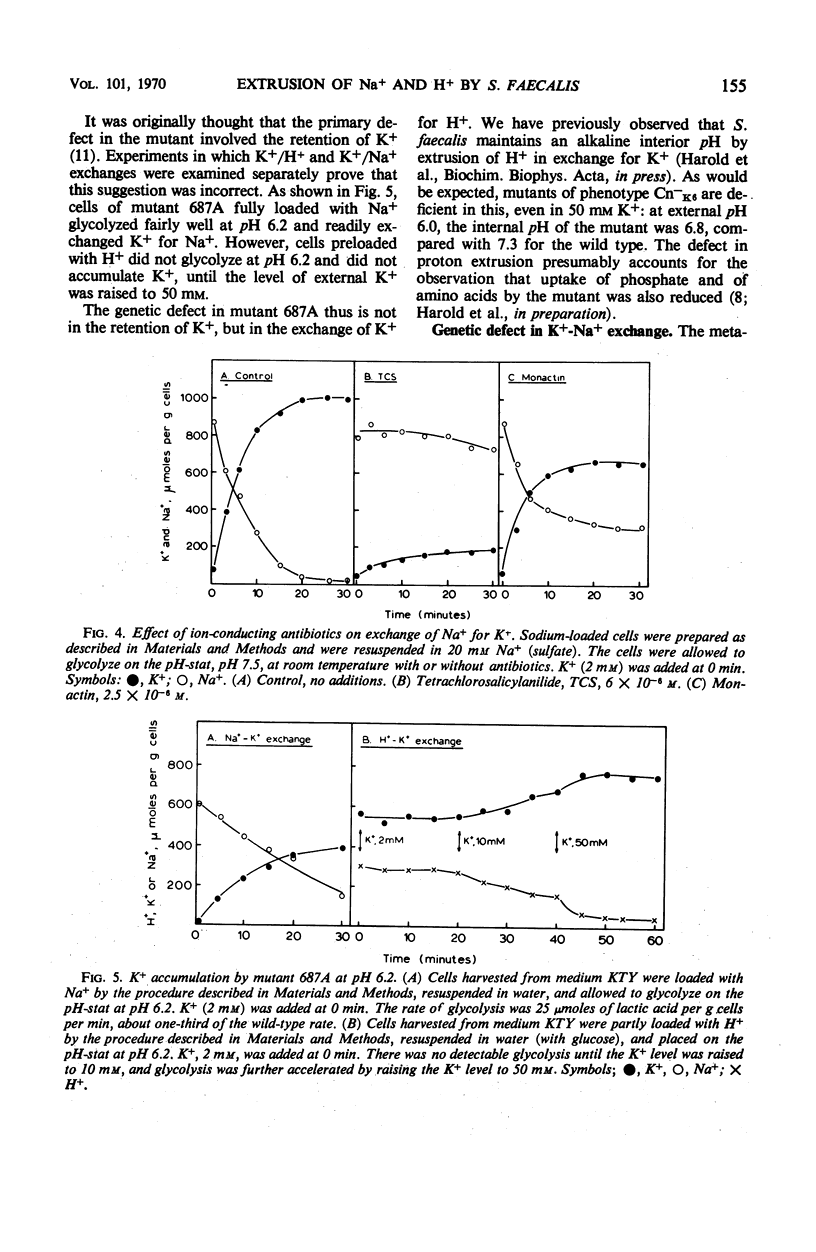
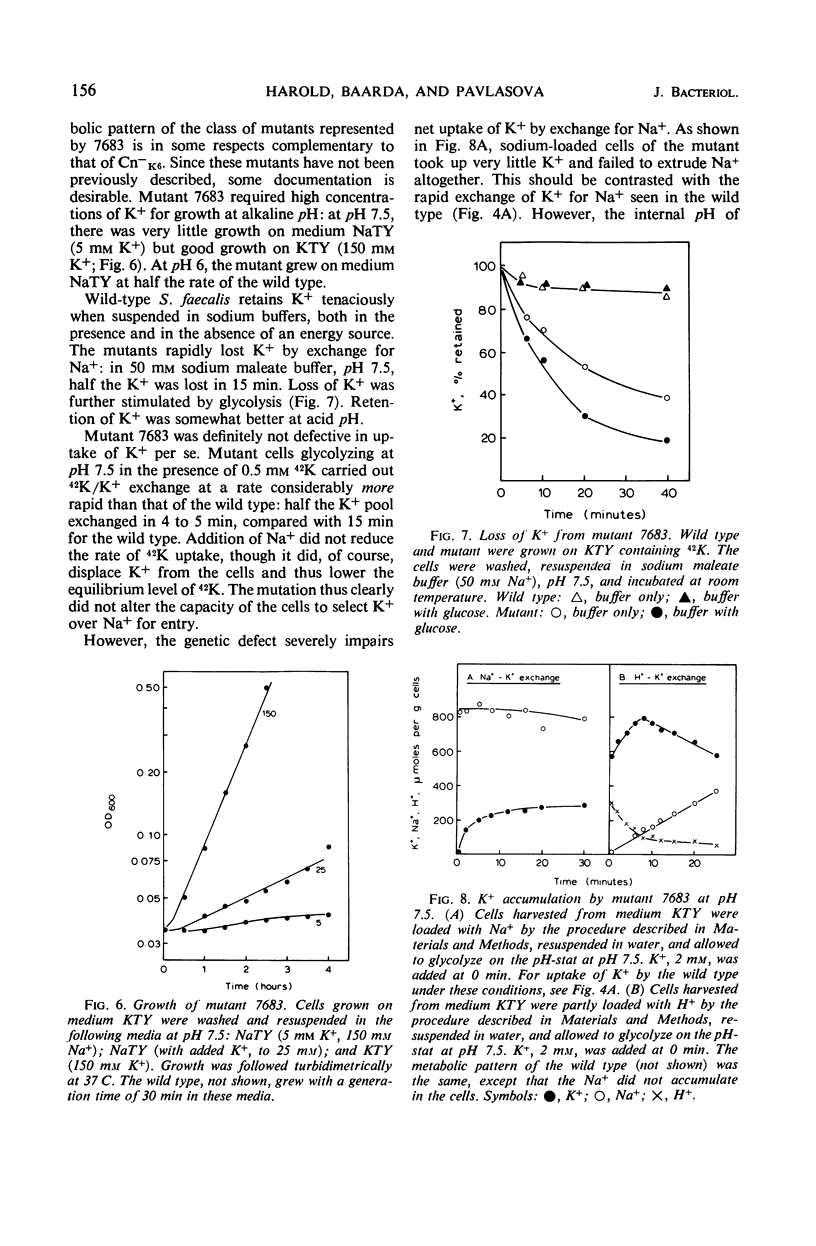
Glycolyzing cells of Streptococcus faecalis accumulate K+ with concurrent extrusion of equivalent amounts of H+ and Na+. An attempt was made to clarify the retionship between the movements of Na+ and K+. Sodium was displaced from cells glycolyzing in the presence of ammonia, diethylamine, tris(hydroxymethyl)aminomethane, and other nitrogenous cations; by contrast, K+ was completely retained. Accumulation of K+ by heterologous exchange for Na+ was not inhibited by antibiotics which facilitate diffusion of K+ across the membrane, but was blocked by proton conductors. The results indicate that extrusion of Na+ and H+ from the cells is a primary, energy-linked process which generates an electrical potential (interior negative); K+ accumulation occurs in response to this potential. Two mutants deficient in K+ accumulation and retention were examined in terms of this model. One mutant is apparently defective in exchange of K+ for H+. In the other mutant, exchange of K+ for Na+ is impaired.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of potassium transport by sodium in a mutant of Streptococcus faecalis. Biochemistry. 1967 Oct;6(10):3107–3110. doi: 10.1021/bi00862a018. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Pressman B. C. The direction of polarity of the mitochondrial trans-membrane potential. Biochim Biophys Acta. 1969 Jan 14;172(1):66–70. doi: 10.1016/0005-2728(69)90092-9. [DOI] [PubMed] [Google Scholar]
- LUBIN M., ENNIS H. L. ON THE ROLE OF INTRACELLULAR POTASSIUM IN PROTEIN SYNTHESIS. Biochim Biophys Acta. 1964 Apr 27;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- LUBOCHINSKY B., MEURY J., STOLKOWSKI J. CIN'ETIQUE DES 'ECHANGES DE POTASSIUM CHEZ L'ESCHERICHIA COLI, SOUCHE B 207, QUI NE PEUT CRO ITRE NORMALEMENT QU'EN PR'ESENCE DE CONCENTRATIONS 'ELEV'EES EN POTASSIUM. C R Hebd Seances Acad Sci. 1964 May 20;258:5106–5109. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Proton-translocation phosphorylation in mitochondria, chloroplasts and bacteria: natural fuel cells and solar cells. Fed Proc. 1967 Sep;26(5):1370–1379. [PubMed] [Google Scholar]
- Pedersen C. J. Ionic complexes of macrocyclic polyethers. Fed Proc. 1968 Nov-Dec;27(6):1305–1309. [PubMed] [Google Scholar]
- Pressman B. C. Ionophorous antibiotics as models for biological transport. Fed Proc. 1968 Nov-Dec;27(6):1283–1288. [PubMed] [Google Scholar]
- ROTHSTEIN A. Role of the cell membrane in the metabolism of inorganic electrolytes by microorganisms. Bacteriol Rev. 1959 Dec;23(4):175–201. doi: 10.1128/br.23.4.175-201.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., EPSTEIN W., SOLOMON A. K. CATION TRANSPORT IN ESCHERICHIA COLI. IV. KINETICS OF NET K UPTAKE. J Gen Physiol. 1963 Nov;47:329–346. doi: 10.1085/jgp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Cation transport in Escherichia coli. I. Intracellular Na and K concentrations and net cation movement. J Gen Physiol. 1961 Nov;45:355–369. doi: 10.1085/jgp.45.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Effects of external cations on the intracellular potential. J Gen Physiol. 1965 Sep;49(1):69–92. doi: 10.1085/jgp.49.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Respiration and the intracellular potential. J Gen Physiol. 1965 Sep;49(1):93–116. doi: 10.1085/jgp.49.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Net uptake of potassium in Neurospora. Exchange for sodium and hydrogen ions. J Gen Physiol. 1968 Sep;52(3):424–443. doi: 10.1085/jgp.52.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. W., Tatum E. L. Potassium transport in Neurospora. 3. Isolation of a transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):184–193. doi: 10.1016/0926-6585(65)90102-0. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Ennis H. L. Ribonucleic acid and protein synthesis in a mutant of Bacillus subtilis defective in potassium retention. J Bacteriol. 1968 Dec;96(6):2035–2042. doi: 10.1128/jb.96.6.2035-2042.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARLENGO M., ABRAMS A. Selective penetration of ammonia and alkylamines into Streptococcus fecalis and their effect on glycolysis. Biochim Biophys Acta. 1963 Apr 2;71:65–77. doi: 10.1016/0006-3002(63)90986-7. [DOI] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]


