Abstract
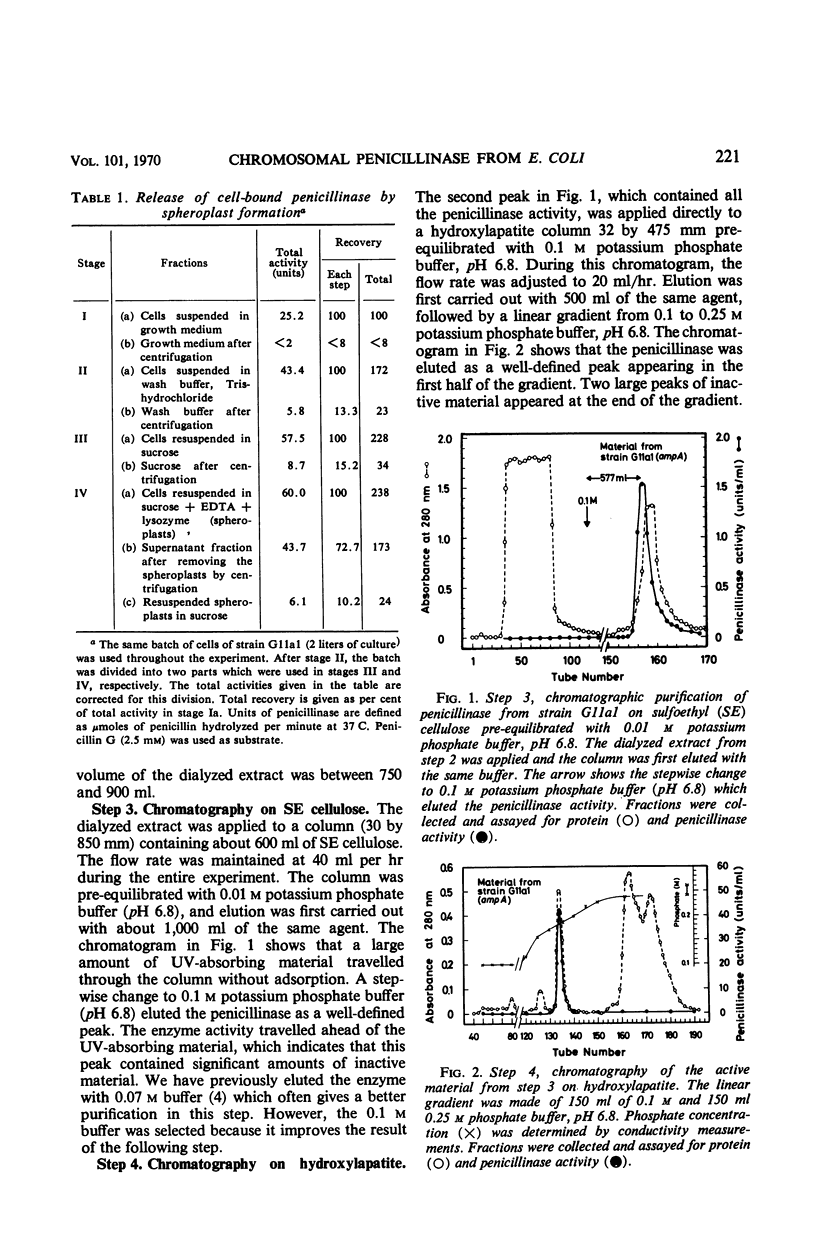
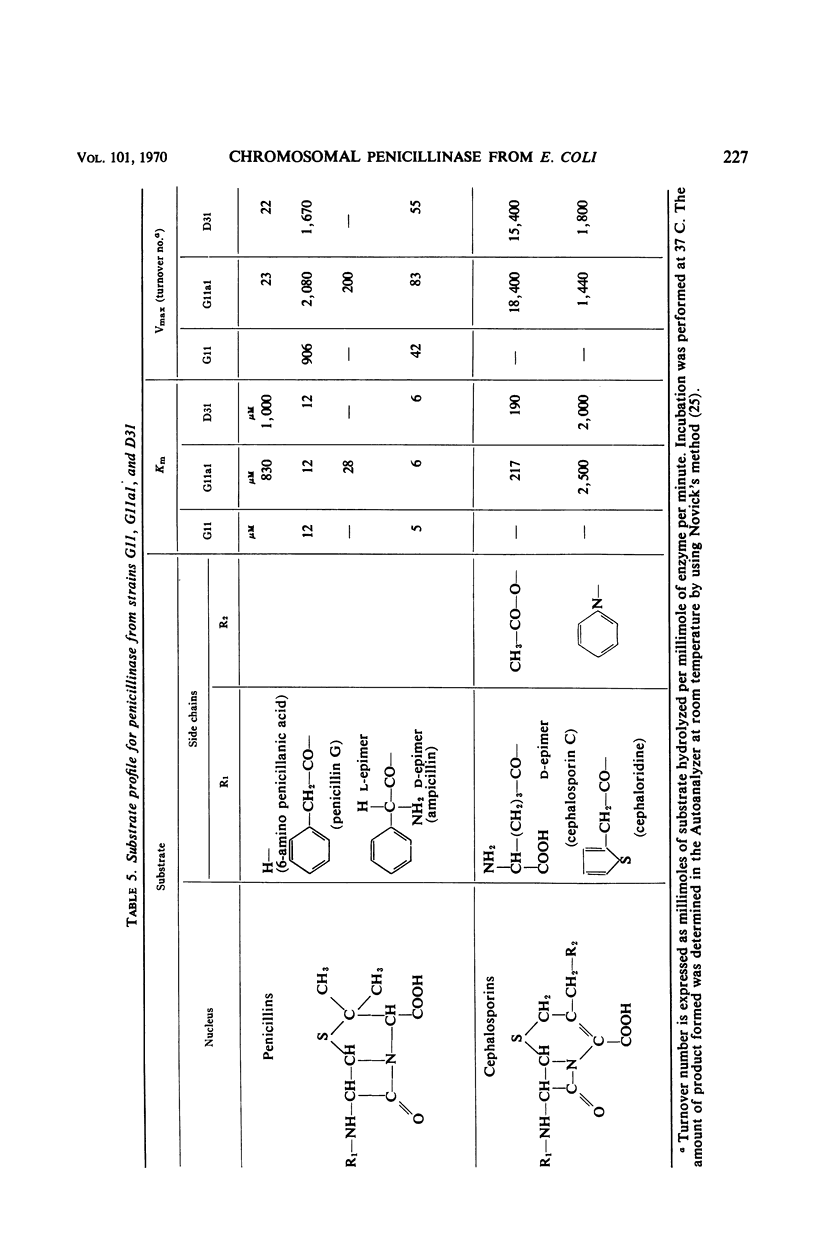
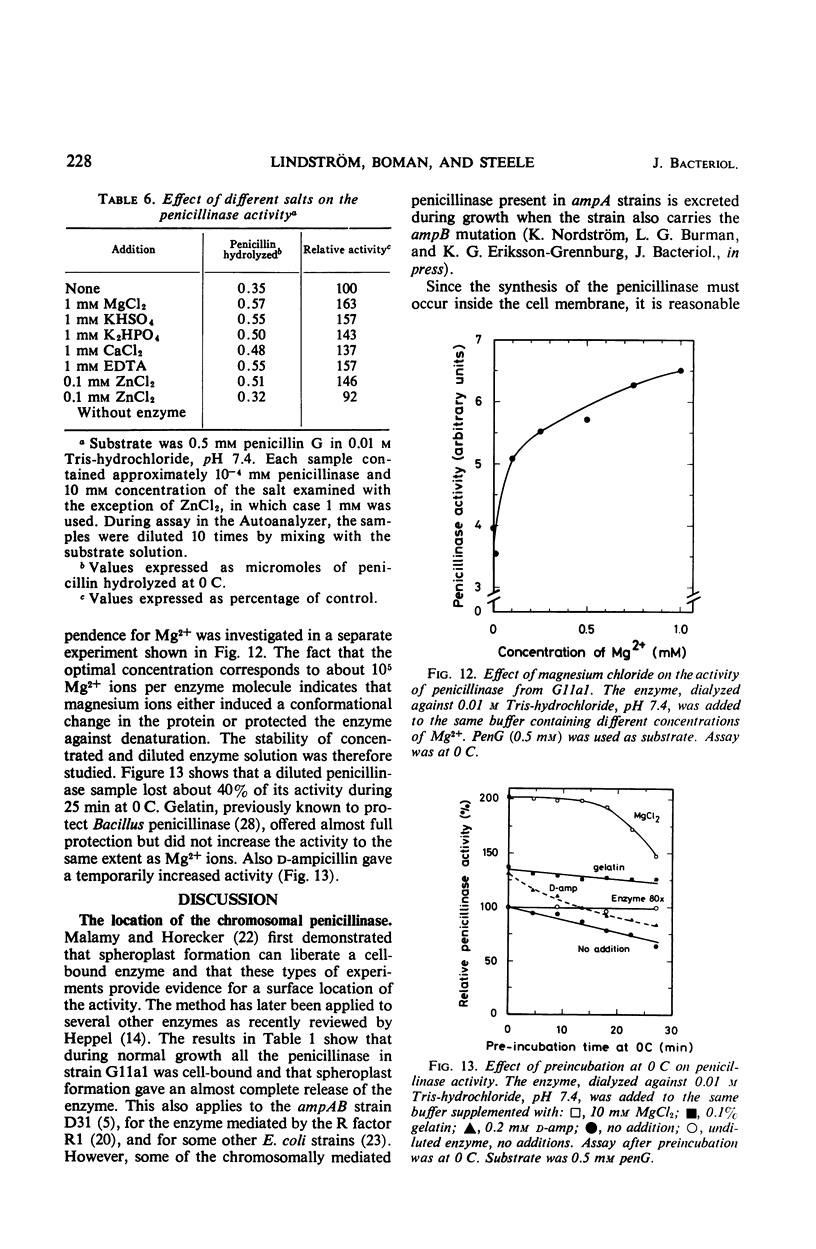
The chromosomally mediated penicillinase present in three strains of Escherichia coli K-12 has been purified and characterized. Two of the strains carried the ampA gene and the third the wild-type allele. The purification involves release of the enzyme by spheroplast formation, dialysis, chromatography on sulfoethyl cellulose, and chromatography on hydroxylapatite. Enzyme from the two mutants appeared homogeneous in polyacrylamide gel electrophoresis. Enzyme from the wild-type strain gave two bands. Immunologically, the enzymes from all three strains were identical. Ultracentrifugation gave a homogeneous peak with a sedimentation coefficient of 3.4S. Gel filtration gave an estimated molecular weight of 29,000. The N-terminal amino acid residue was found to be alanine. Complete amino acid analysis showed a lack of cysteine. Ultraviolet spectra were recorded at three different pH values. The extinction coefficient at 280 nm is 21.0 for a 1% solution at pH 6.8. The optimal pH is 7.3. With enzyme from one of the resistant mutants, the following Km and turnover number values were obtained: for penicillin G, 12 μm and 2,080; for d-ampicillin, 6 μm and 83; for cephalosporin C, 217 μm and 18,400. The effect of different salts on the enzyme activity was tested. Under many conditions the enzyme was found to be unstable.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Meadway R. J. Chemical structure of bacterial penicillinases. Nature. 1969 Apr 5;222(5188):24–26. doi: 10.1038/222024a0. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMAN H. G., ERIKSSON K. G. Penicillin-induced lysis in Escherichia coli. J Gen Microbiol. 1963 Jun;31:339–352. doi: 10.1099/00221287-31-3-339. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. A., Pollock M. R. The genetics of Bacillus licheniformis penicillinase: a preliminary analysis from studies on mutation and inter-strain and intra-strain transformations. J Gen Microbiol. 1965 Oct;41(1):7–21. doi: 10.1099/00221287-41-1-7. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S., SJOQUIST J. Quantitative determination of N-terminal amino acids in some serum proteins. Biochim Biophys Acta. 1960 Dec 4;45:290–296. doi: 10.1016/0006-3002(60)91453-0. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. II. An improved mapping of the ampA gene. Genet Res. 1968 Oct;12(2):147–156. doi: 10.1017/s0016672300011769. [DOI] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hjertén S., Jerstedt S., Tiselius A. Some aspects of the use of "continuous" and "discontinuous" buffer systems in polyacrylamide gel electrophoresis. Anal Biochem. 1965 May;11(2):219–223. doi: 10.1016/0003-2697(65)90008-4. [DOI] [PubMed] [Google Scholar]
- Kasik J. E., Peacham L. Properties of beta-lactamases produced by three species of mycobacteria. Biochem J. 1968 May;107(5):675–682. doi: 10.1042/bj1070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- POLLOCK M. R. THE DIFFERENTIAL EFFECT OF ACTINOMYCIN D ON THE BIOSYNTHESIS OF ENZYMES IN BACILLUS SUBTILIS AND BACILLUS CEREUS. Biochim Biophys Acta. 1963 Sep 17;76:80–93. [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Pollock M. R. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967 Oct 14;4(5571):71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock M. R. The range and significance of variations amongst bacterial penicillinases. Ann N Y Acad Sci. 1968 Jun 14;151(1):502–515. doi: 10.1111/j.1749-6632.1968.tb11910.x. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- SJOQUIST J. Determination of amino acids as phenyl thiohydantoin derivatives. III. Quantitative determination of 3-phenyl-2-thiohydantoins from paper chromatograms. Biochim Biophys Acta. 1960 Jun 17;41:20–30. doi: 10.1016/0006-3002(60)90364-4. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Comparison of the chromosomal and extrachromosomal genetic determinants controlling staphylococcal penicillinase production. Jpn J Microbiol. 1968 Dec;12(4):531–533. doi: 10.1111/j.1348-0421.1968.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]





