Abstract
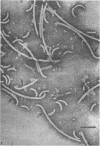
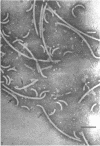
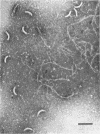
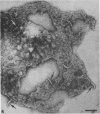
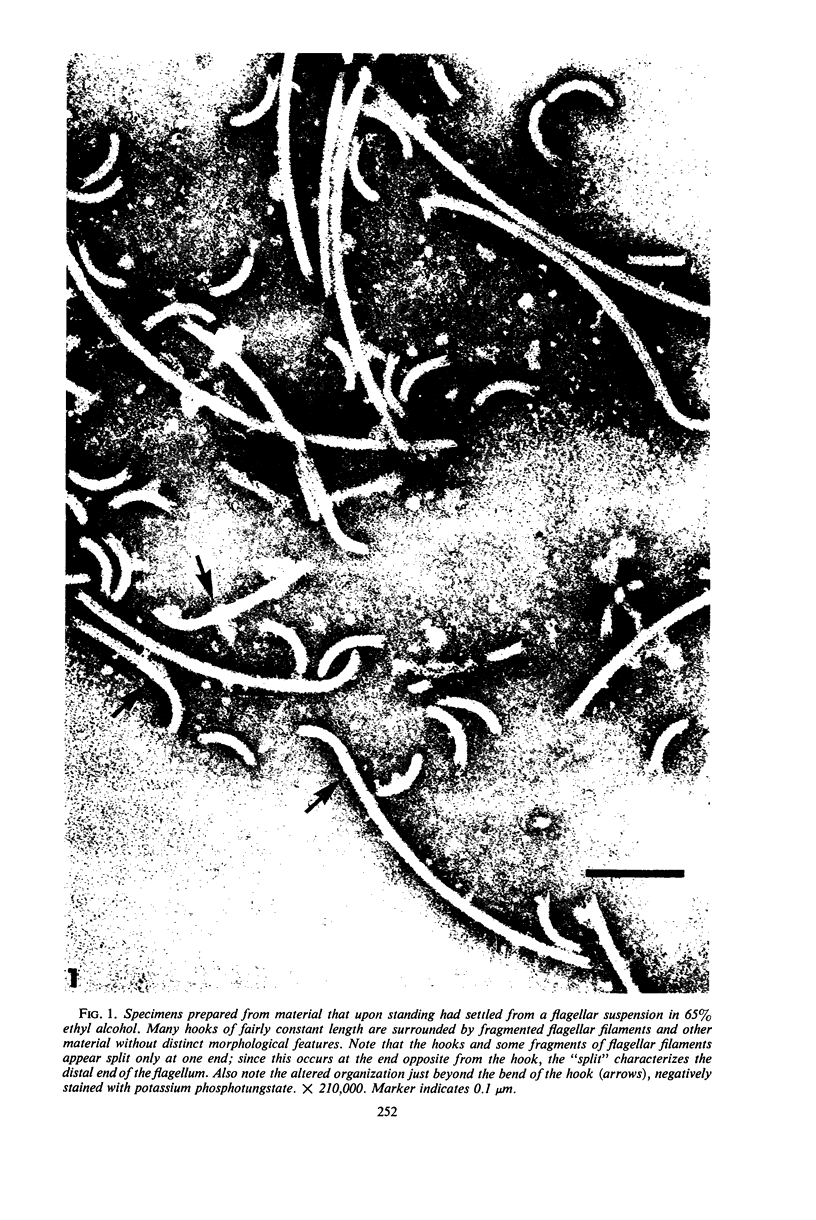
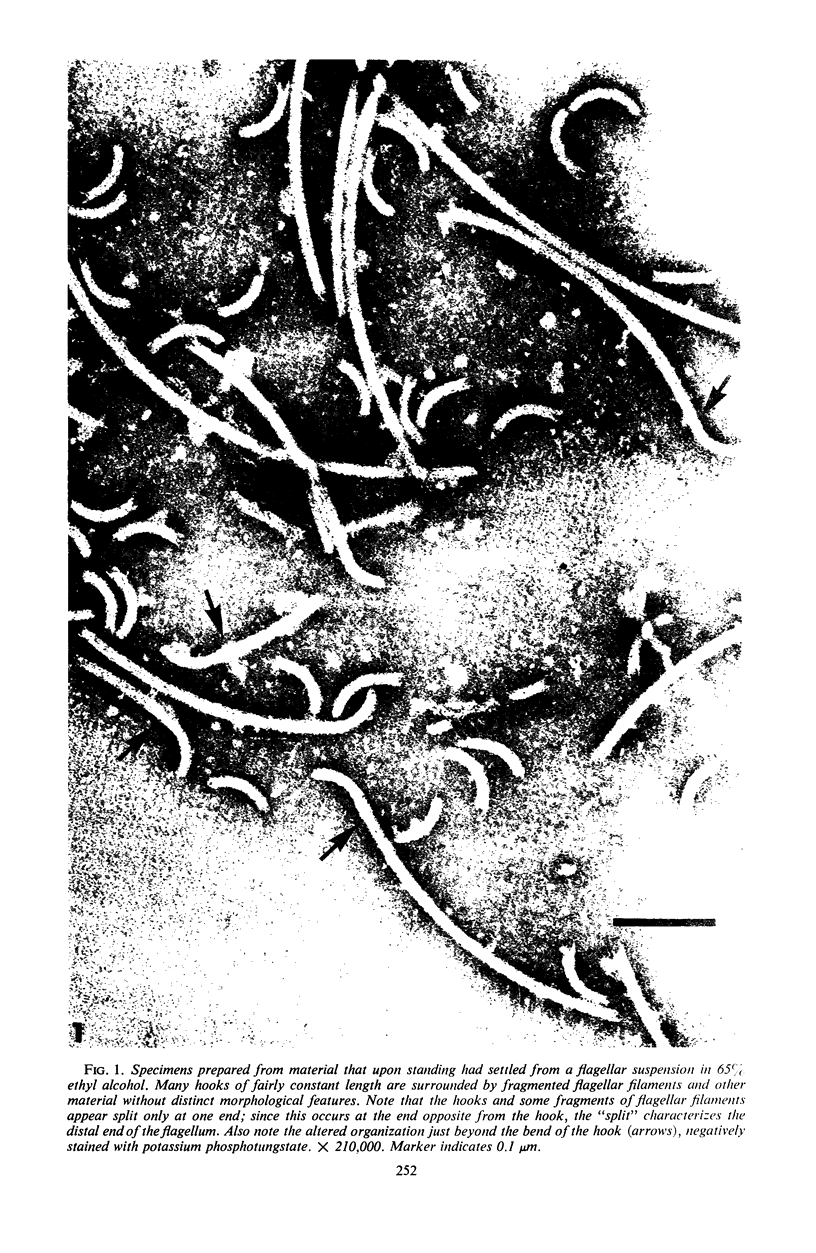
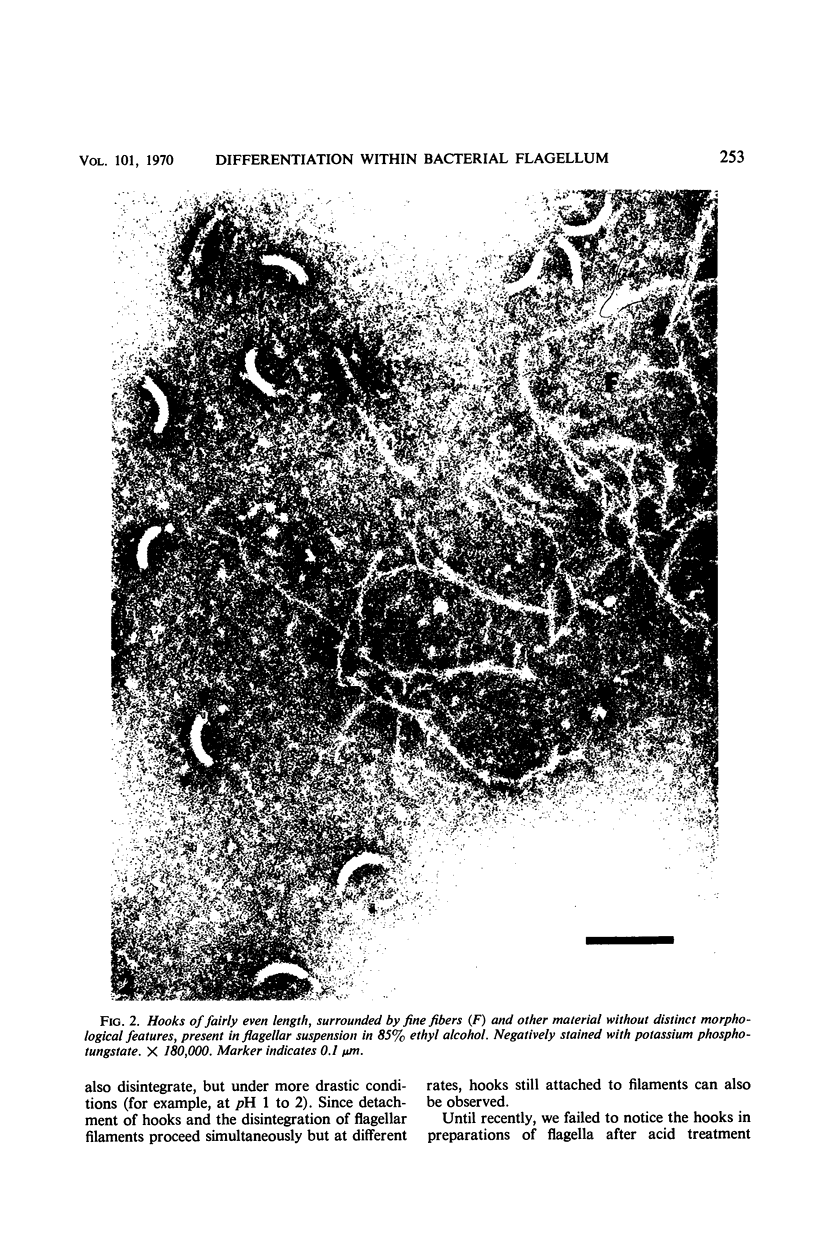
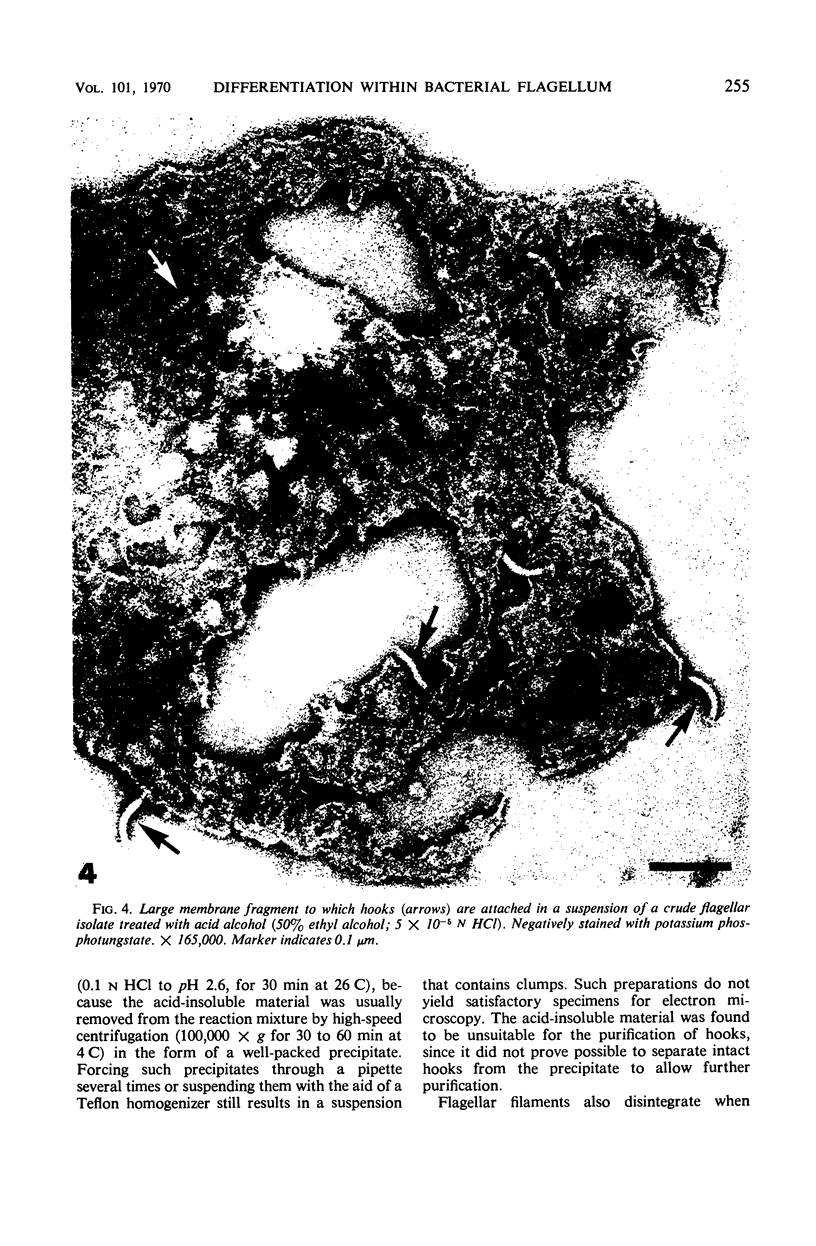
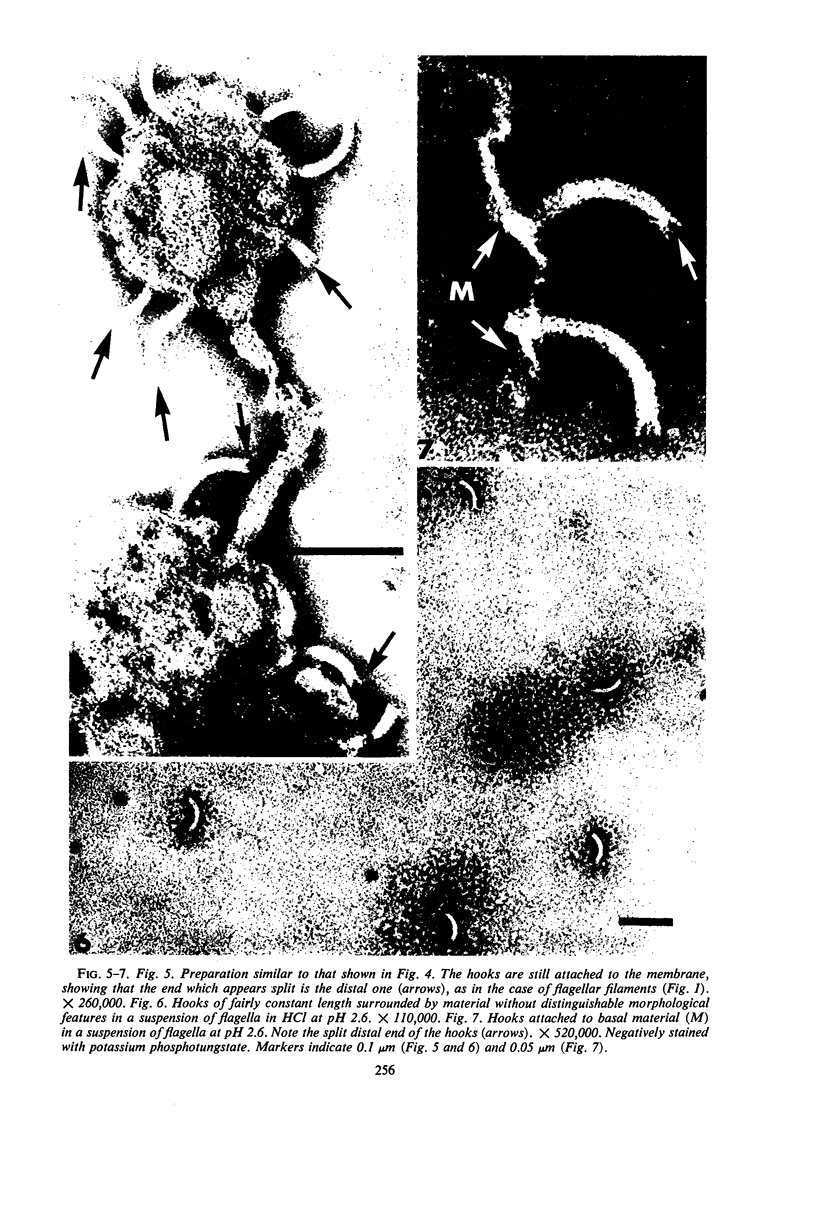
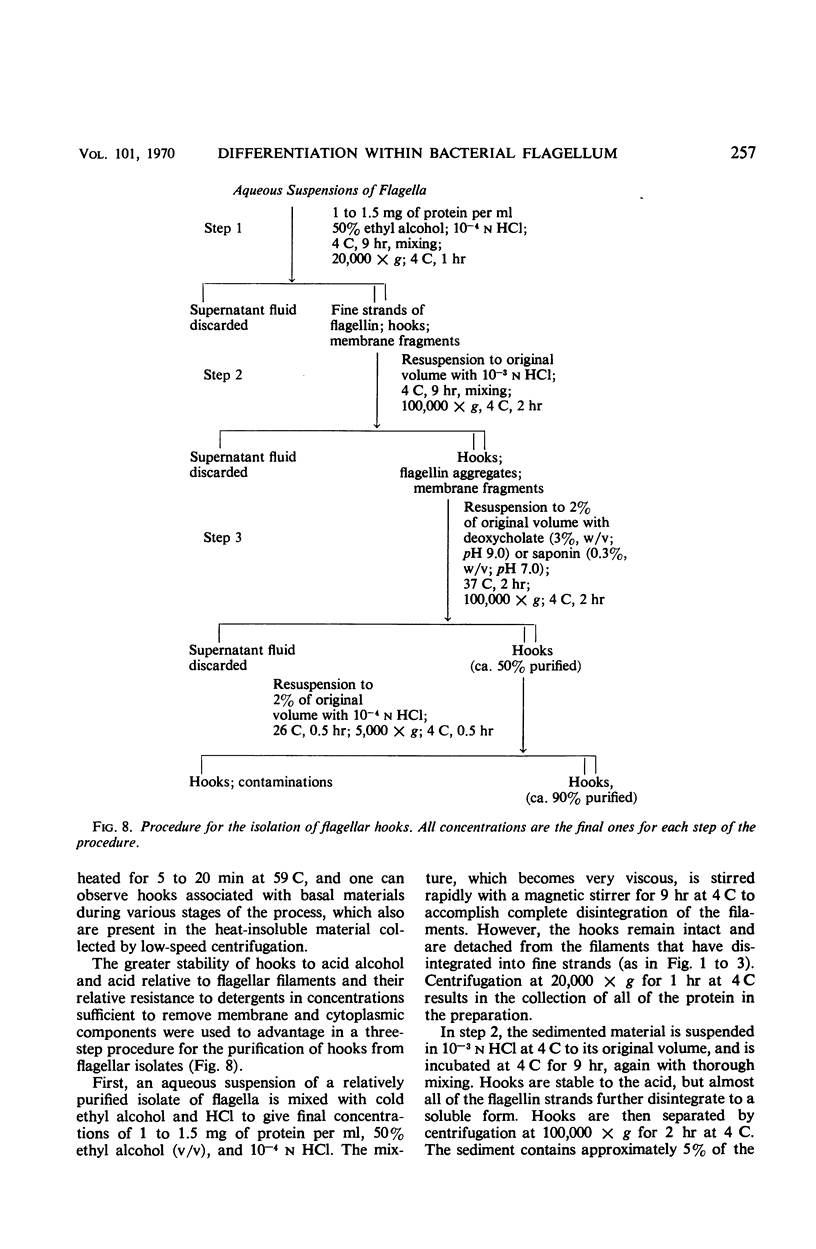
Purified and crude flagellar isolates from cells of Bacillus pumilus NRS 236 were treated with acid, alcohol, acid-alcohol, or heat, and were examined electron microscopically in negatively stained and shadow-cast preparations. Under certain conditions, each of these agents causes the flagella to break between the proximal hooks and the spiral filaments. In such preparations, filaments are seen in various stages of disintegration, whereas hooks of fairly constant length retain their integrity and morphological identity. When crude isolates of flagella are treated under these conditions, the hooks remain attached to membrane fragments or bear basal material. These findings substantiate previous structural observations that led to the view that the proximal hook is a distinct part of the bacterial flagellum and further confirm that the hook is tightly associated with basal material and the cytoplasmic membrane. It appears that the hook is a polarly oriented structure, and that the interactions between the hook and the basal material or the cytoplasmic membrane are different from those between the hook and the filamentous portion of the organelle. Moreover, both types of interaction apparently differ still from those by which the flagellin subunits are held together in the flagellar filament. Hooks were isolated by exploiting the differences in relative stability shown by the various morphological regions of the bacterial flagellum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. BEHAVIOUR OF ACTIVE BACTERIAL ANTIGENS DURING THE INDUCTION OF THE IMMUNE RESPONSE. I. PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA. Nature. 1963 Sep 28;199:1257–1259. doi: 10.1038/1991257a0. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P., REES M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959 Jul 4;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- Abram D., Koffler H., Vatter A. E. Basal structure and attachment of flagella in cells of Proteus vulgaris. J Bacteriol. 1965 Nov;90(5):1337–1354. doi: 10.1128/jb.90.5.1337-1354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram D., Vatter A. E., Koffler H. Attachment and structural features of flagella of certain bacilli. J Bacteriol. 1966 May;91(5):2045–2068. doi: 10.1128/jb.91.5.2045-2068.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. Adsorption of polysomes to bacterial membranes. J Mol Biol. 1966 Feb;15(2):505–514. doi: 10.1016/s0022-2836(66)80124-9. [DOI] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Unidirectional growth of Salmonella flagella in vitro. J Mol Biol. 1968 Jul 14;35(1):227–236. doi: 10.1016/s0022-2836(68)80050-6. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., London J. Basal organelles of bacterial flagella. J Bacteriol. 1967 Aug;94(2):458–465. doi: 10.1128/jb.94.2.458-465.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANDER S. R., KOFFLER H., FOSTER J. F. Physical properties of flagellin from Proteus vulgaris, a study involving the application of the Archibald sedimentation principle. Arch Biochem Biophys. 1960 Sep;90:139–153. doi: 10.1016/0003-9861(60)90625-1. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., KERRIDGE D., HORNE R. W. THE FINE STRUCTURE AND MODE OF ATTACHMENT OF THE SHEATHED FLAGELLUM OF VIBRIO METCHNIKOVII. J Cell Biol. 1963 Aug;18:327–336. doi: 10.1083/jcb.18.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRACE J. B. Some observations on the flagella and blepharoplasts of Spirillum and Vibrio spp. J Gen Microbiol. 1954 Apr;10(2):325–327. doi: 10.1099/00221287-10-2-325. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. A macromolecular mono-layer in the cell wall of Spirillum spec. Biochim Biophys Acta. 1953 Mar;10(3):360–366. doi: 10.1016/0006-3002(53)90266-2. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L., van ITERSON W. Electron microscopical observations on bacterial cytology; a study on flagellation. Biochim Biophys Acta. 1950 Mar;5(1):10–44. doi: 10.1016/0006-3002(50)90144-2. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Van Iterson W., Van Zanten E. N. Basal bodies of bacterial flagella in Proteus mirabilis. II. Electron microscopy of negatively stained material. J Cell Biol. 1966 Dec;31(3):603–618. doi: 10.1083/jcb.31.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Polarity of flagellar growth in salmonella. J Gen Microbiol. 1969 May;56(2):227–239. doi: 10.1099/00221287-56-2-227. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D., HORNE R. W., GLAUERT A. M. Structural components of flagella from Salmonella typhimurium. J Mol Biol. 1962 Apr;4:227–238. doi: 10.1016/s0022-2836(62)80001-1. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., RINKER J. N., KOFFLER H. Purification and and chemical properties of flagellin. Arch Biochem Biophys. 1959 Oct;84:342–362. doi: 10.1016/0003-9861(59)90598-3. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. Simple immunological labelling method for electron microscopy and its application to the study of filamentous appendages of bacteria. Nature. 1967 Jun 10;214(5093):1151–1152. doi: 10.1038/2141151a0. [DOI] [PubMed] [Google Scholar]
- Lowy J. Structure of the proximal ends of bacterial flagella. J Mol Biol. 1965 Nov;14(1):297–299. doi: 10.1016/s0022-2836(65)80251-0. [DOI] [PubMed] [Google Scholar]
- Mitani M., Iino T. Phenocopies of a heteromorphous flageller mutant in Salmonella. J Bacteriol. 1967 Feb;93(2):766–767. doi: 10.1128/jb.93.2.766-767.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEASE P. Some observations upon the development and mode of attachment of the flagella in Vibrio and Spirillum species. Exp Cell Res. 1956 Feb;10(1):234–237. doi: 10.1016/0014-4827(56)90092-1. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. E., Keeler R. F., Bryner J. H. Anatomical features of Vibrio fetus: Electron microscopic survey. J Gen Microbiol. 1966 Jun;43(3):427–438. doi: 10.1099/00221287-43-3-427. [DOI] [PubMed] [Google Scholar]
- TAWARA J. Electron-microscopic study on the flagella of Vibrio comma. J Bacteriol. 1957 Jan;73(1):89–90. doi: 10.1128/jb.73.1.89-90.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAWARA J. MANNER OF ATTACHMENT OF FLAGELLA IN VIBRIO COMMA. J Bacteriol. 1964 Aug;88:531–532. doi: 10.1128/jb.88.2.531-532.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Iterson W., Hoeniger J. F., Van Zanten E. N. Basal bodies of bacterial flagella in Proteus mirabilis. I. Electron microscopy of sectioned material. J Cell Biol. 1966 Dec;31(3):585–601. doi: 10.1083/jcb.31.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegotsky A., Lim F., Foster J. F., Koffler H. Disintegration of flagella by acid. Arch Biochem Biophys. 1965 Aug;111(2):296–307. doi: 10.1016/0003-9861(65)90190-6. [DOI] [PubMed] [Google Scholar]