Abstract
Pten is a negative regulator of the Akt pathway, and its inactivation is believed to be an etiological factor in many tumor types. Pten+/- mice are susceptible to a variety of spontaneous tumor types, depending on strain background. Pten+/- mice, in lung tumor-sensitive and -resistant background strains, were treated with a tobacco carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), to determine whether allelic Pten deletion can cooperate with NNK in carcinogenesis in lung or other tissues. In lung tumor-resistant C57BL/6 Pten+/- or +/+ mice, NNK treatment did not lead to any lung tumors and did not increase the incidence or severity of tumors previously reported for this strain. In contrast, in a lung tumor-susceptible pseudo-A/J strain, there was a dose-dependent increase in lung tumor size in Pten+/- compared with +/+ mice, although there was no increase in multiplicity. No other tumor types were observed in pseudo-A/J Pten+/- mice regardless of NNK treatment. Lung tumors from these Pten+/- mice had K-ras mutations, retained Pten expression and had similar Akt pathway activation as lung tumors from +/+ mice. Therefore, deletion of a single copy of Pten does not substantially add to the lung tumor phenotype conferred by mutation of K-ras by NNK, and there is likely no selective advantage for loss of the second Pten allele in lung tumor initiation.
Introduction
Pten is a phosphatase whose inactivation is believed to be an etiological factor in many human tumor types. Although Pten has homology to protein tyrosine phosphatases, its primary substrates are 3′ phosphoinositides. In this respect, Pten acts as an antagonist to the phosphoinositide 3-kinase (PI3K)-Akt pathway, which is frequently activated and associated with poor prognosis in many tumor types [1]. Pten mutation coupled with loss of heterozygosity is found at a high frequency in endometrial, prostate, and high-grade glial tumors [2]. Mutations occur most frequently but not exclusively within the phosphatase domain of the protein, implicating the effects on PI3K-Akt signaling as a primary tumor suppressor function of Pten.
Genetically engineered mice with germline deletion of a single copy of Pten exhibit some of the same tumor types observed in human patients with germline Pten mutation. Loss or reduction of Pten protein is common in non-small cell lung cancer [3], as is Akt activation [4], but it is unclear whether mutant or decreased Pten is a risk factor for tobacco-related neoplasms. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific nitrosamine, which is the most potent carcinogen in tobacco smoke, has been widely used to induce lung tumors in mice [5]. Both NNK treatment and Pten mutation lead to Akt pathway activation in vitro and in vivo [6,7]. In genetically engineered mouse models, lung-specific deletion of Pten coupled with K-ras mutation led to a more malignant lung tumor phenotype, although Akt is most likely a primary target for mutant K-ras-mediated lung tumorigenesis [4,8,9].
Lung carcinogenesis by NNK in mice is highly strain-dependent, and only lung tumors have been described in susceptible strains. However, other cancers have been associated with tobacco use in humans, and there are no mouse models to investigate these. To assess lung carcinogenesis and potentially other tumors types, we treated Pten+/- mice in a lung tumor-susceptible (pseudo-A/J) or lung tumor-resistant background (C57BL/6) with NNK. Pten+/- pseudo-A/J mice did not show an increase in lung tumor susceptibility, although there was a dose-dependent increase in tumor size. Surprisingly, no other tumors were observed in these mice up to 30 weeks of age. Nearly all lung tumors from NNK-treated Pten+/- and +/+ mice had K-ras mutations in exon 1. Similar Akt pathway activation was observed in both groups, although tumors from Pten+/- mice showed decreased, but not absent, Pten expression. In a resistant strain background, C57BL/6, Pten+/- mice developed adrenal, endometrial, and prostate tumors irrespective of NNK treatment, but no lung tumors. These results indicate that loss of a single copy of Pten does not increase tobacco carcinogen sensitivity, perhaps due to the redundancy of signaling pathways induced by Pten hetero-zygosity and K-ras mutation.
Materials and Methods
Mouse Husbandry and Carcinogen Treatment
Pten+/- mice [10] in a C57BL/6 background (eight backcrosses) were obtained from the Mouse Models of Human Cancer Consortium and had two copies of C57BL/6 K-ras allele as determined by polymerase chain reaction (PCR). These mice were backcrossed twice with A/J mice, which are susceptible to lung tumors induced by NNK treatment. Lung tumor-susceptible mice carried two copies of the wild type A/J K-ras allele and are referred to as pseudo-A/J. Pten+/- pseudo-A/J were crossed with A/J and +/+ and +/- littermates were used for lung tumorigenesis studies. Mice were injected intraperitoneally with NNK (Eagle Pitcher) at 100 mg/kg beginning at 6 weeks of age with either a single dose or three doses at weekly intervals. At 24 weeks after a single dose or 16 weeks after the first of three doses, mice were killed by cervical dislocation, lungs inflated with 10% neutral-buffered formalin, and tissues fixed in the same. The following day, lung lobes were separated and surface lung tumors were counted and measured with a dissecting microscope. Untreated mice were also killed at the same age. Pten+/- mice were also crossed with C57BL/6 mice, and the progenies were used for single-dose NNK carcinogenesis but with an end point at 30 weeks after treatment. Data were analyzed using Prism (GraphPad Software, Inc, San Diego, CA) using unpaired t tests.
Tissues were processed to paraffin, sectioned, and stained with hematoxylin and eosin. All tissues were evaluated by a board-certified veterinary pathologist. Tissues evaluated included adrenal glands, cervical lymph nodes, spleen, thymus, pancreas, kidneys, lungs, heart, liver, bladder, bone marrow, colon, intestines, brain, and male and female reproductive organs.
Pten genotype was determined by PCR from tail clips [11]. Briefly, 5 mm of the tail tip was lysed overnight in 100 µl of 100 mM Tris, pH 8, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, and 100 µg/ml proteinase K. The following day, 300 µl of water was added, and samples were boiled for 10 minutes before PCR on 1 µl for each sample. K-ras allele strain contribution was determined by PCR for Kras_37 [12]. The A/J allele yielded a 91-bp PCR product, whereas the C57BL/6 allele yielded a 128-bp product.
Analysis of K-ras Mutations
K-ras mutations were detected in individual lung tumors using the Surveyor Mutation Detection kit (Transgenomic, Inc., Omaha, NE). After paraffin removal with xylene, tumors were excised from unstained 20-µm sections using razor blades and a dissecting microscope. Tumors were identified on adjacent sections stained with hematoxylin and eosin and were clearly visible on unstained sections. Tumor tissue was digested in 50 µl of 100 mM Tris, pH 8.0, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, and 100 µg/ml proteinase K overnight at 55°C. Approximately 50 µl of water was added, samples were boiled, and 5 µl was used for amplification of K-ras exons 1 and 2 as described previously [13]. Internal primers were used for a second round of amplification to generate single bands for the mutation assay (exon 1, 5′-gtaaggcctgctgaaaatgact-3′ and 5′-gactgtagagcagcgttacct-3′ generating a product of 146 bp; exon 2, 5′-caagtagtaattgatggagaaacctgt-3′ and 5′-caacttaaacccacctataatggtga-3′ generating a product of 178 bp). Tumor PCR products were mixed with PCR product from A/J mouse tail DNA. Cleavage products of approximately 94 and 52 bp were expected for mutations at codon 12 in exon 1 of K-ras, whereas cleavage products of 121 and 67 bp were expected for mutations at codon 61 of exon 2.
Immunohistochemistry for Pten and Akt Pathway Status
Pten, pS473-Akt, and pS235/236 ribosomal S6 expression in lung tumors was determined by immunohistochemistry as described previously [1] with the following exceptions. A Pten rabbit monoclonal antibody (Cell Signaling Technology, Danvers, MA) and an IR800-anti-rabbit secondary (Invitrogen, Carlsbad, CA) was used to quantify Pten. Samples were counterstained with the DNA stain, To-Pro3-iodide (Invitrogen), which emits in the IR700 channel, and were scanned on an Odyssey (Licor Biosciences, Lincoln, NE) infrared scanner at 21-µm resolution. Relative Pten expression was determined as the relative ratio of Pten signal to To-pro3-iodide signal. Tumor locations were verified by comparison to adjacent sections stained with hematoxylin and eosin. Immunohistochemistry for pS473-Akt and pS235/236 ribosomal S6 protein (antibodies 3787 and 4857; Cell Signaling Technology) were performed as described previously. Staining was scored as absent (0), mild (1), moderate (2), or strong (3). Staining index was determined by summing the (fraction of cells staining) x (intensity) as described previously [1].
Results
Development of Lung Tumor-Susceptible and -Resistant Pten+/- Mice
Pten+/- mice were treated with a tobacco carcinogen to determine whether allelic loss of Pten might confer increased risk for tumors with a known tobacco-related component. However, strain-specific effects of genetically modified mice are common [14] and Pten+/- mice develop a different spectrum of tumors depending on strain background [10,15–17]. In addition, both spontaneous and NNK-induced lung tumors are strain-dependent, with A/J being the most susceptible strain, conferred by the K-ras allele [18,19]. To make pure C57BL/6 Pten+/- mice susceptible to lung tumorigenesis, they were bred with A/J mice, and Pten+/- mice carrying the susceptible A/J K-ras allele were selected for this study. These pseudo-A/J and C57BL/6 Pten+/- mice were treated with NNK because we expected different tumor spectrums for each background strain.
Lung Tumorigenesis in Pseudo Pten+/- A/J Mice
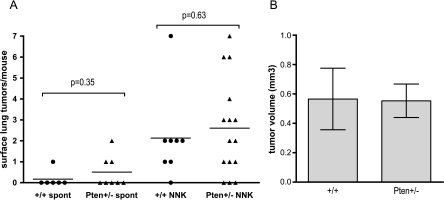
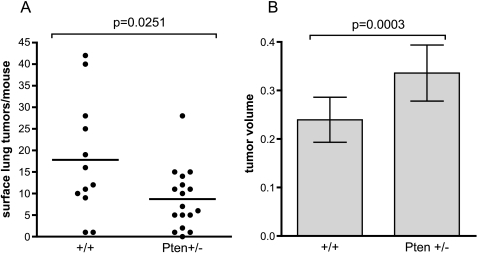
Consistent with the inbred A/J strain, pseudo-A/J mice developed both spontaneous and NNK-induced lung tumors, although with somewhat lower multiplicity than observed for inbred A/J mice (Figure 1) [1]. At 24 weeks after a single dose of NNK (at 30 weeks of age), nearly all +/+ and Pten+/- mice had developed at least one surface lung tumor, which is in contrast to the spontaneous group, most of which had no tumors. Multiplicity was similar for +/+ and Pten+/- mice within both the NNK-treated and untreated groups (Figure 1A). In addition, there was no significant difference in tumor size between the Pten+/- and +/+ mice (Figure 1B). Due to the low lung tumor multiplicity in this study (approximately 2/mouse), a new cohort was treated with three doses of NNK at weekly intervals and killed 16 weeks after the first dose (at 22 weeks of age; Figure 2). More lung tumors were observed in both +/+ and Pten+/- groups with three doses of NNK when compared with a single dose (a nine-fold increase for +/+). However, in the three-dose group, Pten+/- mice developed significantly fewer lung tumors compared with +/+ littermates (Figure 2A). Individual tumor size, however, was increased in the Pten+/- mice and averaged 40% larger by volume (Figure 2B). There were no differences between lung tumor incidence, multiplicity, and size between male and female Pten+/- mice (data not shown).
Figure 1.
Pten+/- mice do not have enhanced susceptibility to either spontaneous or NNK-induced lung tumors. Surface lung tumor multiplicity and tumor size from pseudo-A/J Pten+/- and +/+ littermates 24 weeks after treatment with 100 mg/kg NNK at 6 weeks of age or untreated. (A) Each point represents a single mouse. (B) Tumor volume is per individual tumor. The spontaneous cohort consisted of two +/+ females, four +/+ males, two Pten+/- females, six Pten+/- males. The NNK-treated cohort consisted of four +/+ females, four +/+ males, eight Pten+/- females, and seven Pten+/- males. All P values in this and subsequent figures were obtained using an unpaired t test.
Figure 2.
Pten+/- mice develop larger but increased numbers of lung tumors with three doses of NNK. Surface lung tumor multiplicity and tumor size from pseudo-A/J Pten+/- and +/+ littermates 16 weeks after initial treatment with three doses of 100 mg/kg NNK administered weekly starting at 6 weeks of age. (A) Each point represents a single mouse. (B) Tumor volume is per individual tumor and is presented as mean ± 95% confidence interval for 214 and 148 lung tumors from Pten+/+ and +/- mice, respectively. This cohort consisted of 4 +/+ females, 8 +/+ males, 6 Pten+/- females, and 11 Pten+/- males.
Lack of Lung Tumors in Pten+/- Mice in a Resistant Strain Background
C57BL/6 is one of the most lung tumor-resistant strains of mice [18]. To determine possible strain-dependent effects of NNK, mice in a C57BL/6 background (more than eight backcrosses with C57BL/ 6) were treated with a single dose of NNK using an end point at 30 weeks after treatment. No lung tumors were observed in any of these mice, reflecting the relative lung tumor resistance of the background strain (data not shown).
Other Tumor Types in Pten+/- Mice
Because loss of Pten expression is associated with other tumor types in addition to lung tumors, a panel of tissues was examined in Pten+/- and +/+ mice in both the pseudo-A/J and C57BL/6 backgrounds. Because Pten+/- mice are prone to spontaneous tumors depending on background strain, it was thought that tumors not previously described for Pten+/- mice might arise in the pseudo-A/J background. In addition, NNK-induced tumors might arise in tissues other than the lungs for both strains. For these analyses, we included tissues of known tumor development for Pten+/- mice as well as tissues where human tumors have a documented tobacco contribution (2004 Surgeon General's Report—The Health Consequences of Smoking). These included adrenal glands, cervical lymph nodes, spleen, thymus, pancreas, kidneys, lungs, heart, liver, bladder, bone marrow, colon, intestines, brain, and male and female reproductive organs. No tumors other than lung tumors were observed for either the Pten+/- or +/+ mice in the pseudo-A/J background with either one or three doses of NNK, at 24 or 16 weeks after NNK injection, respectively. However, 1 of 14 pseudo-A/J Pten+/- mice showed a malignant lung adenocarcinoma, whereas no carcinomas were observed in the 11 +/+ littermate lungs evaluated. This contrasted with the C57BL/6 background in which NNK-treated Pten+/- mice did not develop lung tumors.
Fewer Pten+/- males than expected were obtained in the C57BL/6 background, which is consistent with previous reports [15]. Consequently, the C57BL/6 NNK-treated Pten+/- group consisted of mostly females. All Pten+/- mice in this background developed at least one tumor, with or without NNK treatment. Most of these tumors have been described previously in Pten+/- mice (Table 1). NNK treatment did not alter tumor/hyperplasia incidence or severity in Pten+/- mice, although one of nine NNK-treated mice developed a liver hemangiosarcoma, a malignant tumor type not observed in the spontaneous group or described previously in Pten+/- mice. Of 13 C57BL/6 Pten+/- mice in the spontaneous and NNK groups, 12 developed adrenal phenochromocytoma by 36 weeks of age. Prostate tumors were observed in all Pten+/- males (1/1 with four independent adenomas in the NNK group, 2/2 with adenoma in spontaneous group, one of these with a concurrent carcinoma). Severe lymphoid hyperplasia of the cervical lymph nodes was observed in all Pten+/- mice in both NNK and spontaneous groups. Although lymph nodes were generally 1 cm or larger in diameter (data not shown), none were classified as lymphoma on histologic evaluation. In addition, areas of severe uterine hyperplasia were observed in some Pten+/- females in the absence of any endometrial tumors, which have been described for Pten+/- mice in a CD-1 background [15].
Table 1.
Pathologic Findings from Pten+/- and +/+ Mice.
| Spontaneous | NNK | |||
| Pten+/- | +/+ | Pten+/- | +/+ | |
| C57BL/6 background | ||||
| Benign pheochromocytoma | 3/4 | 0/5 | 8/9 | 0/5 |
| Malignant pheochromocytoma | 1/4 | 0/5 | 0/9 | 0/5 |
| Prostate adenoma | 2/2 | 0/5 | 1/1 | 0/5 |
| Prostate carcinoma | 1/2 | 0/5 | 0/1 | 0/5 |
| Liver hemangiosarcoma | 0/4 | 0/5 | 1/9 | 0/5 |
| Severe lymphoid hyperplasia | 4/4 | 1/5 | 9/9 | 0/5 |
| Severe uterine hyperplasia | 1/2 | 0/5 | 3/7 | 0/5 |
In the pseudo-A/J strain background, mice were treated with three doses of NNK and evaluated at 16 weeks after the first injection. In the C57BL/6 strain background, mice were treated with a single dose of NNK and evaluated at 30 weeks after treatment. Values indicate the number of mice affected/total mice evaluated. For prostate and uterine lesions, denominators reflect only males and females, respectively. For prostate, only one of two C57BL/6 Pten+/- males treated with NNK had tissue for evaluation.
From surface lung tumor counts for this group, 16/17 Pten+/- and 14/14 Pten+/+ had visible lung tumors (Figure 2).
Pten Expression Is Retained in Lung Tumors from Pten+/- Mice
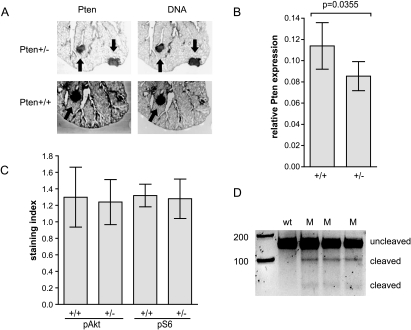
Loss of Pten hemizygosity has been reported in several spontaneous tumor types in Pten+/- mice [10,15,16,20]. Complete or allelic loss of Pten has been described in human lung tumors [21], although no lung tumors have been reported in Pten+/- mice. In NNK-treated Pten+/- mice, no increase in lung tumor number was observed, and Pten expression was not lost in 17 lung tumors from eight pseudo-A/J Pten+/- mice (Figure 3). Lung tumors (Figure 3B) and normal lung tissue (data not shown) from Pten+/- mice showed decreased Pten protein expression when compared with +/+ mice. Although Pten can down-regulate the Akt pathway, decreased Pten levels in Pten+/- mice did not seem to affect Akt pathway activation in lungs, because pAkt and pS6 levels were similar in tumors (Figure 3C) and normal tissue (data not shown) from +/+ and Pten+/- mice.
Figure 3.
Pten and K-ras status of lung tumors from NNK-treated or untreated Pten+/- and +/+ mice. (A) Representative Pten immunohistochemistry staining (visualized using an IR800 secondary antibody) and DNA staining (To-pro3-iodide). Slides were scanned at 1x using a 21-µm resolution on an Odyssey infrared scanner, and portions were enlarged to show detail. Each panel shows approximately one-third of the left lung lobe. Tumors are indicated with arrows. (B) Relative Pten staining from Pten+/- and +/+, NNK-induced lung tumors. (C) Equivalent Akt pathway activation in lung tumors from Pten+/- and +/+ mice as determined by pS473-Akt and pS235/ 236 S6 staining. Quantitation methods are described in the Materials and Methods section. (D) K-ras mutation was determined by Surveyor Mutation Detection kit. K-ras mutation in exon 1 is seen as cleaved DNA in lanes marked “M.” Nonmutated K-ras does not show any cleaved product in the wild type (wt).
Lung Tumors from Pten+/- Mice Harbor K-ras Mutations at the Expected Frequency
NNK-induced lung tumors in A/J mice have been linked to mutations in K-ras [22]. Because Pten allelic loss is associated with lung tumors in humans, it was thought that Pten+/- mice might develop NNK-induced lung tumors in the absence of K-ras mutations. However, similar frequencies of K-ras mutations were found in lung tumors from Pten+/- mice compared with tumors from their +/+ littermates. Of 17 lung tumors from eight Pten+/- mice, 15 carried a K-ras exon 1 mutation; whereas of 20 lung tumors from eight +/+ littermates, 15 carried a K-ras exon 1 mutation (Figure 3A; data not shown). This is similar to published results for exon 1 K-ras mutations in lung tumors from NNK-treated A/J mice [23].
Discussion
Although lung cancer is the major tobacco-associated neoplasm, tobacco use contributes to other tumor types in humans. However, in mice, no tobacco carcinogen-associated tumors have been described in tissues other than in the lung. Pten alteration, by loss of heterozygosity, mutation, or methylation, is common in human lung cancer [9,21] and in other tumor types [24]. Humans and mice with germline Pten alteration develop multiple spontaneous tumor types [6,25], but sensitivity to tobacco carcinogens is unknown. For these reasons, Pten+/- mice were investigated as a potential model for tobacco carcinogen-induced tumorigenesis in lung and in other tissues. Consistent with previous publications, we found that spontaneous tumor types in Pten+/- mice were highly dependent on strain background. The pseudo-A/J strain was resistant to all tumors previously described for Pten+/- mice and did not show any spontaneous tumors other than in the lungs. In contrast, no lung tumors were observed in C57BL/6 Pten+/- mice, although nearly all developed adrenal tumors, consistent with previous studies for a mixed C57BL/6 background [17]. NNK had no effect on incidence or severity of non-lung lesions in C57BL/6 mice, although a single Pten+/- NNK-treated mouse developed a liver hemangiosarcoma, a tumor type not previously described for Pten+/- mice. This implies that NNK does not substantially cooperate with Pten allelic loss in tumorigenesis. However, the considerable difference in tumor spectrum between the two strains highlights the effects of other, as yet undetermined genes that can modify tumorigenesis in Pten+/- mice. Such phenotypic variation is consistent with the phenotypic spectrum of patients with Cowden disease characterized by germline Pten mutations. In Cowden disease, symptoms and severity vary widely [25], which may reflect the contribution of polymorphisms in other loci that modify the effect of Pten mutation in specific tissues. Overall, strain differences in genetically engineered mice are common and highlight the need to investigate mouse models of human disease in multiple background strains [14].
Decreased or absent Pten protein expression is common in human lung adenocarcinomas and is associated with poor prognosis [3,26]. However, biallelic mutation or loss of Pten have not been associated with K-ras mutations in lung tumors. In mice, homozygous deletion of Pten in lung type II cells led to malignant lung tumors starting at 1 year of age and in an increase in urethane-induced lung tumors [8], which have been shown previously to be highly associated with K-ras mutation [27]. In another study, homozygous Pten deletion in bronchial epithelium Clara cells did not lead to any spontaneous lung tumors in mice up to 1 year of age but did increase the malignancy of lung tumors in a genetic model of mutant K-ras [9]. The two studies described involved K-ras mutation coincident with total deletion of Pten in only specific lung cell types and lung tumors. This is in contrast to the studies described here where one copy of Pten was retained in all tissues and was not lost in any mutant K-ras-containing lung tumors. The absence of increased lung tumorigenesis in Pten+/- mice supports the hypothesis that decreased Pten expression is involved in the progression of lung tumors rather than in their initiation. This is in contrast to the effects observed in other tissues such as the intestines, breast, and endometrium, where Pten heterozygosity leads to increased tumors [10,15] with frequent loss of the remaining Pten allele [15]. Although normal lung tissues from pseudo-A/J Pten+/- mice show decreased expression of Pten protein compared with +/+ lungs, NNK-induced lung tumors in these mice retain the expression of Pten (Figure 3). Because NNK did not increase lung tumor incidence in Pten+/- mice, this suggests either that the loss of the second Pten allele does not occur at a high frequency in lung tissue or that there is no selective advantage for total Pten loss in mouse lung tumors. Because homozygous Pten deletion seems to enhance the malignancy of mutant K-ras harboring lung tumors [8,9], it is more likely that loss of the second copy of Pten is an infrequent event in mouse lung tumors. However, the increased size of lung tumors in the three-dose NNK pseudo-A/J group suggests that even deletion of a single copy of Pten may enhance the growth of tumors in the presence of other genetic changes.
The Akt pathway is considered an important target for Pten with loss of Pten phosphatase activity leading to increased PIP3, which activates Akt. Mutant K-ras activates PI3K, which also leads to increased PIP3 and Akt activation. Recently, homozygous deletion of PI3K was shown to prevent K-ras-induced lung tumors, suggesting that Akt pathway activation is required for K-ras-driven lung tumorigenesis [28]. Inhibition of downstream Akt signaling, with the mTOR inhibitor rapamycin, decreased NNK-induced lung tumors by 90% [1]. Here, Pten+/- and +/+ lung tumors showed similar Akt pathway activation (Figure 3) suggesting that K-ras mutation alone is enough to activate the pathway to initiate lung tumorigenesis and that deletion of a single copy of Pten does not amplify this signal.
In inbred A/J mice, NNK-induced lung adenocarcinomas were not observed until 25 weeks after treatment [29], and we have found no adenocarcinomas among NNK-induced lung tumors from 15 inbred A/J mice [1]. Although only observed in one mouse, the presence of a malignant lung adenocarcinoma in a Pten+/- mouse at only 16 weeks after treatment suggests that loss of even a single allele of Pten may lead to more aggressive tumors or shorter latency to adenocarcinoma after treatment with a tobacco carcinogen. This is also supported by the larger lung tumor size in Pten+/- mice treated with three doses of NNK. Studies are ongoing to address whether allelic loss of Pten can lead to increased lung adenocarcinomas at later times and whether this could be accompanied by the total loss of Pten.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
References
- 1.Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila RI, Dennis PA. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res. 2007;13:2281–2289. doi: 10.1158/1078-0432.CCR-06-2570. [DOI] [PubMed] [Google Scholar]
- 2.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 3.Marsit CJ, Zheng S, Aldape K, Hinds PW, Nelson HH, Wiencke JK, Kelsey KT. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is con-stitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 5.Hecht SS, Chen CB, Hirota N, Ornaf RM, Tso TC, Hoffmann D. Tobacco-specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J Natl Cancer Inst. 1978;60:819–824. doi: 10.1093/jnci/60.4.819. [DOI] [PubMed] [Google Scholar]
- 6.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, Moghaddam S, Evans CM, Li H, Cai WW, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–1127. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouse Models of Human Cancers Consortium Repository B6.129-Ptentm1Rps PCR Protocol. Available at: http://mouse.ncifcrf.gov/protocols.asp?ID=01XH3& p_allele=Pten%3Ctm1Rps%3E&prot_no=1.
- 12.Mouse Genome Informatics, author. Molecular Probes and Clones. Available at: http://www.informatics.jax.org/searches/probe.cgi?803644.
- 13.Hollander MC, Philburn RT, Patterson AD, Velasco-Miguel S, Friedberg EC, Linnoila RI, Fornace AJ., Jr Deletion of XPC leads to lung tumors in mice and is associated with early events in human lung carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13200–13205. doi: 10.1073/pnas.0503133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linder CC. Genetic variables that influence phenotype. ILAR J. 2006;47:132–140. doi: 10.1093/ilar.47.2.132. [DOI] [PubMed] [Google Scholar]
- 15.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 16.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 17.You MJ, Castrillon DH, Bastian BC, O'Hagan RC, Bosenberg MW, Parsons R, Chin L, DePinho RA. Genetic analysis of Pten and Ink4a/Arf interactions in the suppression of tumorigenesis in mice. Proc Natl Acad Sci USA. 2002;99:1455–1460. doi: 10.1073/pnas.022632099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan J, Barker PE, Nesbitt MN, Ruddle FH. KRAS2 as a genetic marker for lung tumor susceptibility in inbred mice. J Natl Cancer Inst. 1987;79:1351–1357. [PubMed] [Google Scholar]
- 19.Lin L, Festing MF, Devereux TR, Crist KA, Christiansen SC, Wang Y, Yang A, Svenson K, Paigen B, Malkinson AM, et al. Additional evidence that the K-ras protooncogene is a candidate for the major mouse pulmonary adenoma susceptibility (Pas-1) gene. Exp Lung Res. 1998;24:481–497. doi: 10.3109/01902149809087382. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Podsypanina K, Liu X, Crane A, Tan LK, Parsons R, Varmus HE. Deficiency of Pten accelerates mammary oncogenesis in MMTV-Wnt-1 transgenic mice. BMC Mol Biol. 2001;2:2. doi: 10.1186/1471-2199-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virmani AK, Gazdar AF. Tumor suppressor genes in lung cancer. Methods Mol Biol. 2003;222:97–115. doi: 10.1385/1-59259-328-3:097. [DOI] [PubMed] [Google Scholar]
- 22.Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, Anderson MW. Relationship between the formation of promutagenic adducts and the activation of the K-ras protooncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res. 1989;49:5305–5311. [PubMed] [Google Scholar]
- 23.Devereux TR, Belinsky SA, Maronpot RR, White CM, Hegi ME, Patel AC, Foley JF, Greenwell A, Anderson MW. Comparison of pulmonary O6-methylguanine DNA adduct levels and Ki-ras activation in lung tumors from resistant and susceptible mouse strains. Mol Carcinog. 1993;8:177–185. doi: 10.1002/mc.2940080308. [DOI] [PubMed] [Google Scholar]
- 24.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 25.Lopiccolo J, Ballas MS, Dennis PA. PTEN hamartomatous tumor syndromes (PHTS): rare syndromes with great relevance to common cancers and targeted drug development. Crit Rev Oncol Hematol. 2007;63:203–214. doi: 10.1016/j.critrevonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt over-expression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Pan YH, Nuzum EO, Hanson LA, Beer DG. Ki-ras activation and expression in transformed mouse lung cell lines. Mol Carcinog. 1990;3:279–286. doi: 10.1002/mc.2940030508. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110α is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 29.Belinsky SA, Devereux TR, Foley JF, Maronpot RR, Anderson MW. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;52:3164–3173. [PubMed] [Google Scholar]