Abstract
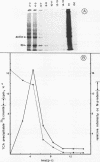
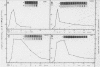
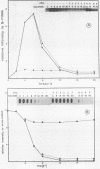
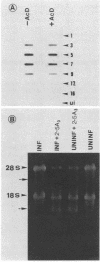
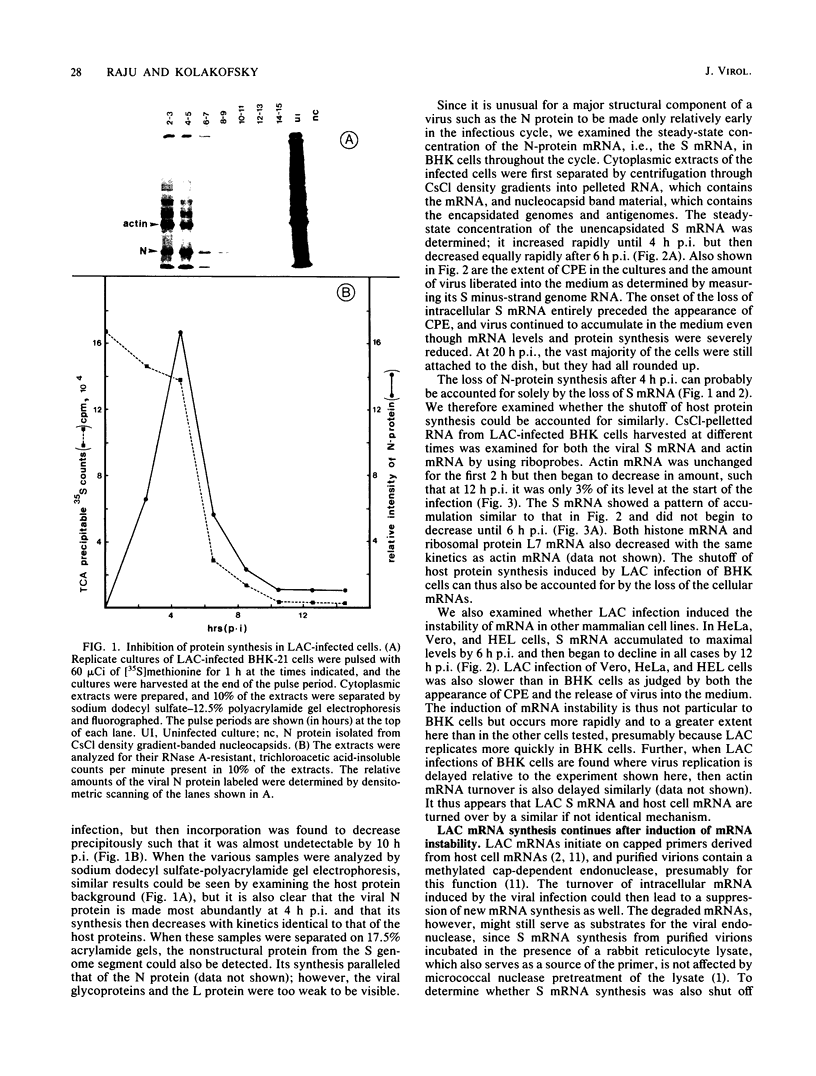
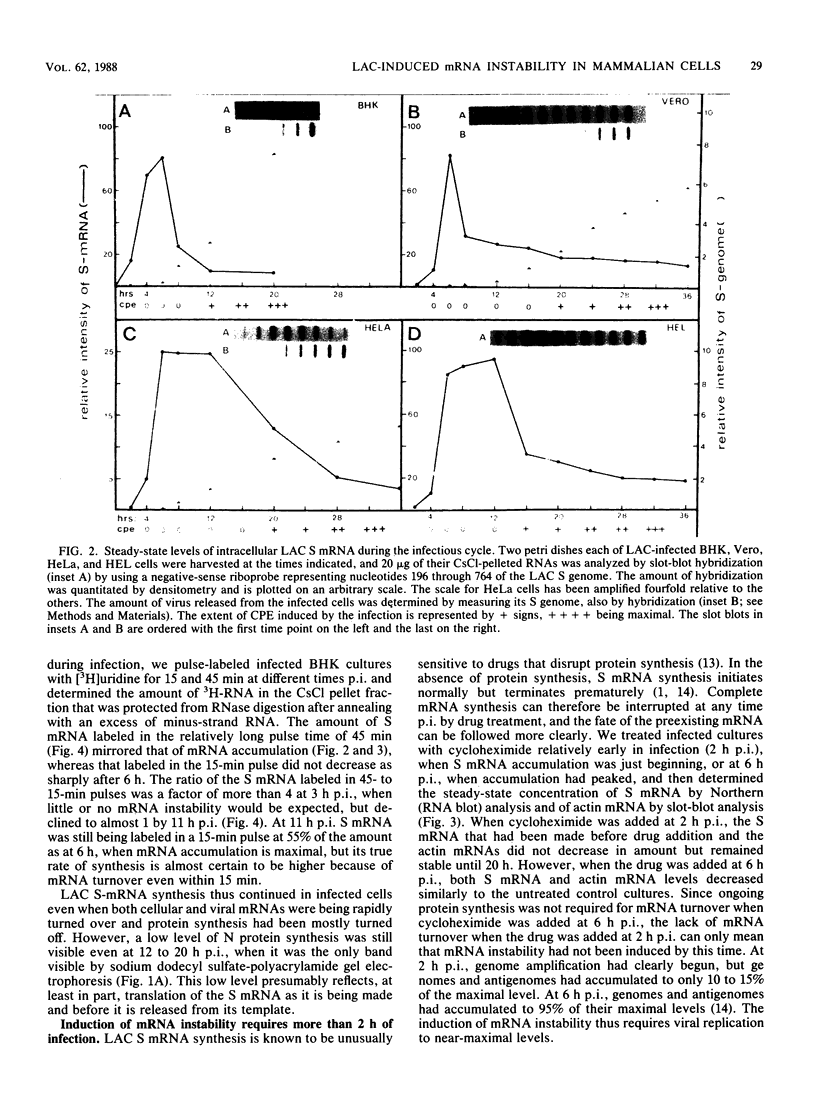
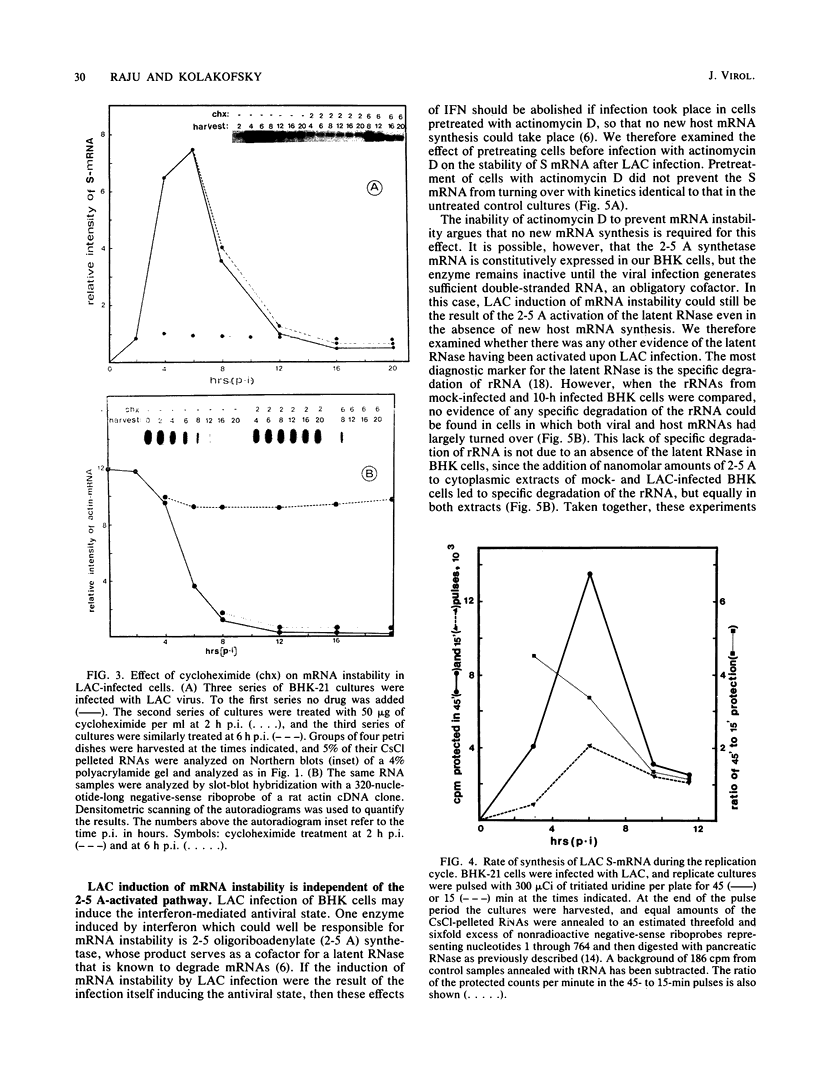
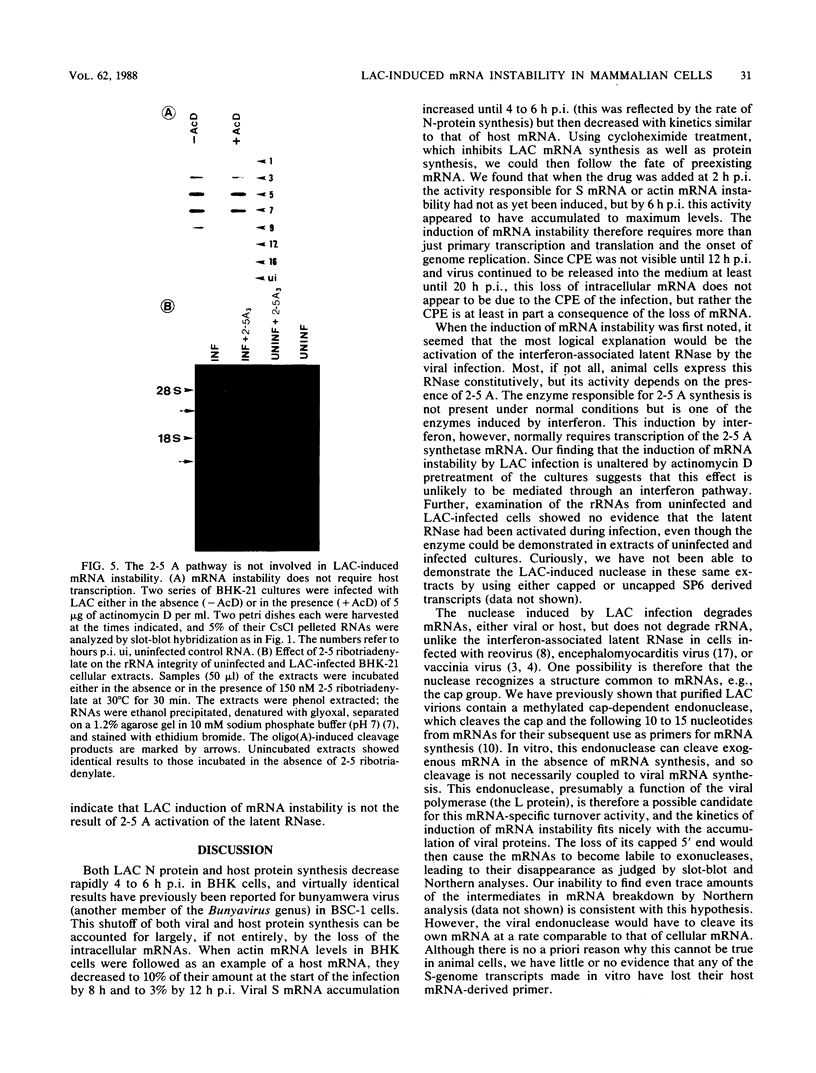
La Crosse virus infection of BHK cells leads to a dramatic shutoff of not only host protein synthesis but also viral protein synthesis later in infection. This shutoff can be accounted for by the loss of the cytoplasmic cellular and viral mRNAs. The induction of mRNA instability requires extensive virus replication, since when cycloheximide is added early in infection the preexisting viral and cellular mRNAs do not decrease upon incubation of the cultures. Pretreatment of the cultures with actinomycin D does not affect the ability of La Crosse virus infection to induce mRNA instability, and examination of the rRNAs shows no evidence of specific degradation due to activation of the interferon-associated latent RNase. The induction of mRNA instability therefore does not appear to operate through an interferon pathway. Viral mRNA synthesis, on the other hand, is not turned off during infection, and the cap-dependent endonuclease involved in viral mRNA initiation may be responsible for the mRNA instability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellocq C., Raju R., Patterson J., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vitro studies. J Virol. 1987 Jan;61(1):87–95. doi: 10.1128/jvi.61.1.87-95.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Benavente J., Paez E. Effect of interferon on integrity of vaccinia virus and ribosomal RNA in infected cells. Virology. 1984 Apr 15;134(1):40–51. doi: 10.1016/0042-6822(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Goswami B. B., Sharma O. K. Degradation of rRNA in interferon-treated vaccinia virus-infected cells. J Biol Chem. 1984 Feb 10;259(3):1371–1374. [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha R. F., Condit R. C. Characterization of a temperature-sensitive mutant of vaccinia virus reveals a novel function that prevents virus-induced breakdown of RNA. J Virol. 1985 Nov;56(2):395–403. doi: 10.1128/jvi.56.2.395-403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington T. H., Pringle C. R., McCrae M. A. Bunyamwera virus-induced polypeptide synthesis. J Virol. 1977 Oct;24(1):397–400. doi: 10.1128/jvi.24.1.397-400.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Inhibitors of protein synthesis inhibit both La Crosse virus S-mRNA and S genome syntheses in vivo. Virus Res. 1986 Jul;5(1):1–9. doi: 10.1016/0168-1702(86)90061-4. [DOI] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1987 Jan;61(1):96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987 Mar;61(3):667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schek N., Bachenheimer S. L. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J Virol. 1985 Sep;55(3):601–610. doi: 10.1128/jvi.55.3.601-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. H., Cayley P. J., Knight M., Gilbert C. S., Kerr I. M. Control of the ppp(a2'p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982 May;124(1):131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Silverman R. H., Skehel J. J., James T. C., Wreschner D. H., Kerr I. M. rRNA cleavage as an index of ppp(A2'p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983 Jun;46(3):1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. H. Vector-virus relationships. Prog Clin Biol Res. 1983;123:57–66. [PubMed] [Google Scholar]
- Turell M. J., LeDuc J. W. The role of mosquitoes in the natural history of California serogroup viruses. Prog Clin Biol Res. 1983;123:43–55. [PubMed] [Google Scholar]