Abstract
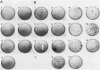
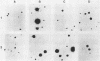
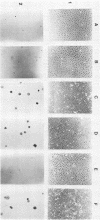
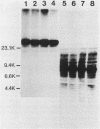
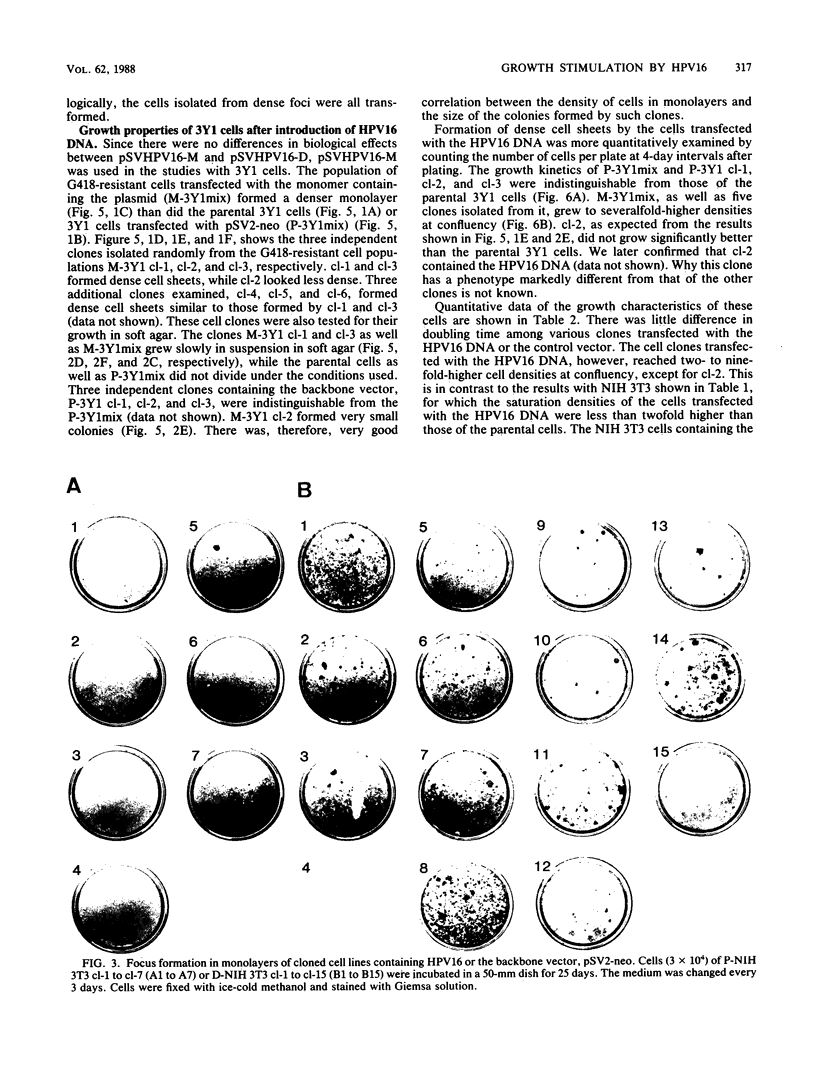
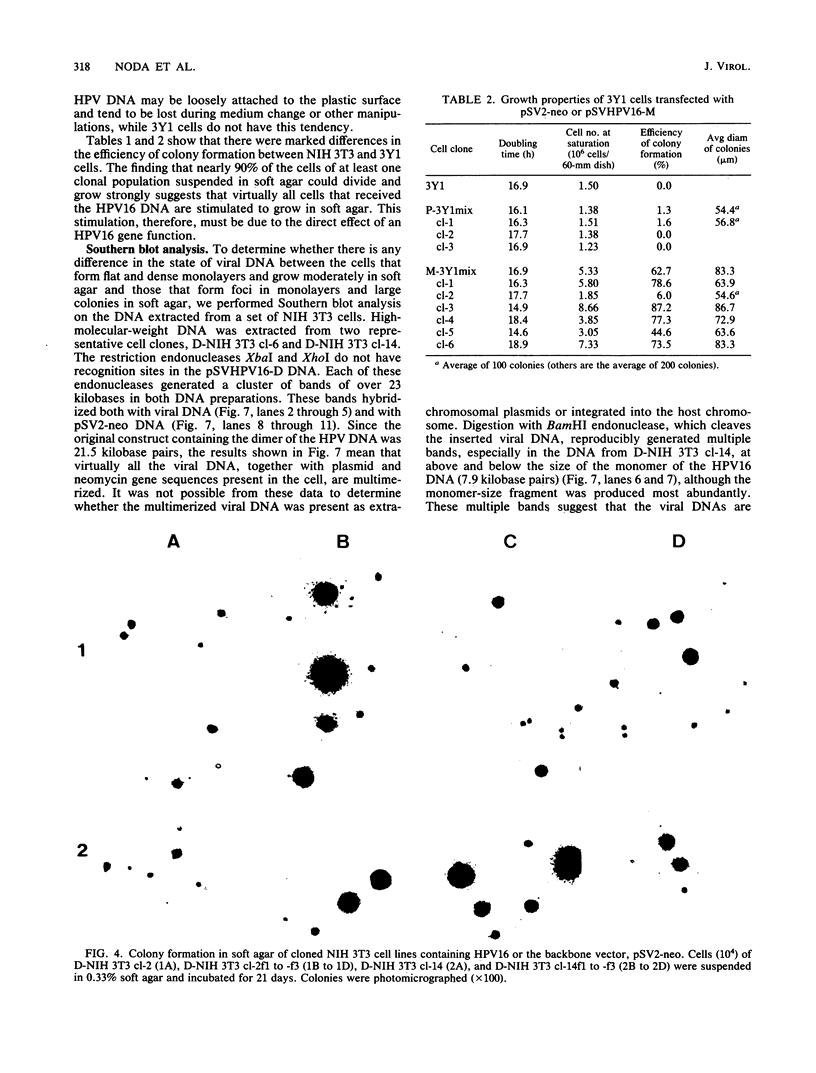
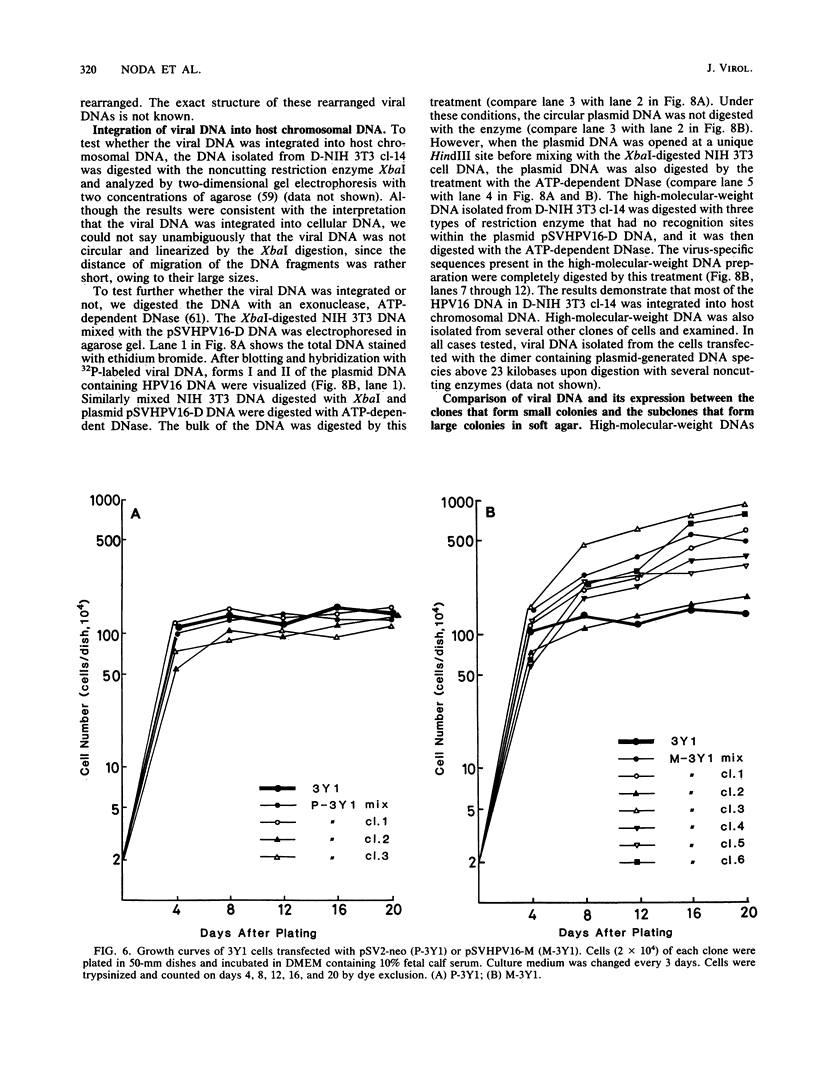
Alteration of the growth properties of the established murine fibroblast cell lines NIH 3T3 and 3Y1 was studied in monolayer cultures and in cells suspended in semisolid medium after introduction of a cloned human papillomavirus type 16 (HPV16) DNA. HPV 16 DNA stimulated both cell lines to grow beyond their saturation densities in monolayer cultures without any apparent morphological changes or tendency to pile up. These cells were also stimulated to grow in soft agar. Since essentially all the cells that received the viral gene were stimulated to grow, the growth-stimulatory activity of HPV16 appeared to be due to the direct effect of a viral gene function. The NIH 3T3 cells showed an additional change in growth properties upon prolonged incubation of dense monolayers of cells containing the HPV16 DNA; morphologically recognizable dense foci appeared at a frequency of about 10(-3). These cells, when cloned from the foci, grew more rapidly in soft agar than the parental cells and were morphologically transformed. In other words, there were two sequential steps in cell transformation induced by HPV16. Practically all the viral DNAs were present in the cells as large rearranged multimers and were integrated into host chromosomal DNA. There was no obvious difference in the state of viral DNA in the cells of the original clone or the three subclones derived from it as dense foci. There was no difference in the amount or the number of viral RNA species expressed in the cells at these two stages. The secondary changes in the growth properties of NIH 3T3 cells appear to be due to some cellular alterations.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M. S., Jarrett W. F. Papillomavirus infection in cattle: viral and chemical cofactors in naturally occurring and experimentally induced tumours. Ciba Found Symp. 1986;120:117–135. doi: 10.1002/9780470513309.ch9. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Moar M. H., Sartirana M. L., Kennedy I. M., Jarrett W. F. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. 1985 Jul;4(7):1819–1825. doi: 10.1002/j.1460-2075.1985.tb03856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Ikenberg H., Richart R. M., Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984 Apr 5;310(14):880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Anderson C., Hunter T., Kaplan P. L. Transmission of the polyoma virus middle T gene as the oncogene of a murine retrovirus. Nature. 1984 Apr 19;308(5961):748–750. doi: 10.1038/308748a0. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grisoni M., Meneguzzi G., de Lapeyrière O., Binétruy B., Rassoulzadegan M., Cuzin F. The transformed phenotype in culture and tumorigenicity of Fischer rat fibroblast cells (FR3T3) transformed with bovine papilloma virus type 1. Virology. 1984 Jun;135(2):406–416. doi: 10.1016/0042-6822(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., McNeil P. E., Grimshaw W. T., Selman I. E., McIntyre W. I. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978 Jul 20;274(5668):215–217. doi: 10.1038/274215a0. [DOI] [PubMed] [Google Scholar]
- Jat P. S., Cepko C. L., Mulligan R. C., Sharp P. A. Recombinant retroviruses encoding simian virus 40 large T antigen and polyomavirus large and middle T antigens. Mol Cell Biol. 1986 Apr;6(4):1204–1217. doi: 10.1128/mcb.6.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Yoshiike K. Human papillomavirus type 16 transformation of rat 3Y1 cells. Jpn J Cancer Res. 1987 Feb;78(2):103–108. [PubMed] [Google Scholar]
- Kidd J. G., Rous P. CANCERS DERIVING FROM THE VIRUS PAPILLOMAS OF WILD RABBITS UNDER NATURAL CONDITIONS. J Exp Med. 1940 Mar 31;71(4):469–494. doi: 10.1084/jem.71.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A. T., Lancaster W. D., Temple G. F. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. J Virol. 1986 Apr;58(1):225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Genetic analysis of bovine papillomavirus type 1 trans-acting replication factors. J Virol. 1985 Mar;53(3):955–965. doi: 10.1128/jvi.53.3.955-965.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura T., Kanda T., Furuno A., Yoshikawa H., Kawana T., Yoshiike K. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J Virol. 1986 Jun;58(3):979–982. doi: 10.1128/jvi.58.3.979-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Campion M. J., Clarkson P. K., Chesters P. M., Jenkins D., Singer A. Prevalence of human papillomavirus type 16 DNA sequences in cervical intraepithelial neoplasia and invasive carcinoma of the cervix. Br J Obstet Gynaecol. 1985 Nov;92(11):1101–1105. doi: 10.1111/j.1471-0528.1985.tb03019.x. [DOI] [PubMed] [Google Scholar]
- Noda T., Satake M., Robins T., Ito Y. Isolation and characterization of NIH 3T3 cells expressing polyomavirus small T antigen. J Virol. 1986 Oct;60(1):105–113. doi: 10.1128/jvi.60.1.105-113.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Satake M., Yamaguchi Y., Ito Y. Cooperation of middle and small T antigens of polyomavirus in transformation of established fibroblast and epithelial-like cell lines. J Virol. 1987 Jul;61(7):2253–2263. doi: 10.1128/jvi.61.7.2253-2263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomaviruses. Ciba Found Symp. 1986;120:157–174. doi: 10.1002/9780470513309.ch11. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Pater A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology. 1985 Sep;145(2):313–318. doi: 10.1016/0042-6822(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Rous P., Kidd J. G. THE CARCINOGENIC EFFECT OF A VIRUS UPON TARRED SKIN. Science. 1936 May 15;83(2159):468–469. doi: 10.1126/science.83.2159.468. [DOI] [PubMed] [Google Scholar]
- Sarver N., Rabson M. S., Yang Y. C., Byrne J. C., Howley P. M. Localization and analysis of bovine papillomavirus type 1 transforming functions. J Virol. 1984 Nov;52(2):377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Lowy D. R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Shope R. E., Hurst E. W. INFECTIOUS PAPILLOMATOSIS OF RABBITS : WITH A NOTE ON THE HISTOPATHOLOGY. J Exp Med. 1933 Oct 31;58(5):607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- Tsunokawa Y., Takebe N., Kasamatsu T., Terada M., Sugimura T. Transforming activity of human papillomavirus type 16 DNA sequence in a cervical cancer. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2200–2203. doi: 10.1073/pnas.83.7.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunokawa Y., Takebe N., Nozawa S., Kasamatsu T., Gissmann L., zur Hausen H., Terada M., Sugimura T. Presence of human papillomavirus type-16 and type-18 DNA sequences and their expression in cervical cancers and cell lines from Japanese patients. Int J Cancer. 1986 Apr 15;37(4):499–503. doi: 10.1002/ijc.2910370405. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O., Stevens J. G. Variable-sized free episomes of Shope papilloma virus DNA are present in all non-virus-producing neoplasms and integrated episomes are detected in some. Proc Natl Acad Sci U S A. 1982 Feb;79(3):790–794. doi: 10.1073/pnas.79.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Tsuda T., Fujimoto S., Toda M., Kato K., Maekawa Y., Umeno M., Anai M. Purification of small polydisperse circular DNA of eukaryotic cells by use of ATP-dependent deoxyribonuclease. Gene. 1983 Dec;26(2-3):317–321. doi: 10.1016/0378-1119(83)90205-6. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Burkhardt A. L., Doniger J., DiPaolo J. A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986 Feb;57(2):572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Doniger J., DiPaolo J. A. Differential early viral gene expression in two stages of human papillomavirus type 16 DNA-induced malignant transformation. Mol Cell Biol. 1987 Jun;7(6):2165–2172. doi: 10.1128/mcb.7.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]