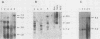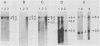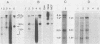Abstract
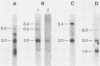
Leukemogenic mink cell focus-forming (MCF) viruses of AKR mice are believed to originate in thymic tissue via recombination between ecotropic, xenotropiclike, and endogenous MCF-related murine leukemia virus (MuLV) sequences. We have previously used a synthetic 16-base-pair MCF env-specific oligomer probe to identify subgenomic MCF-related mRNAs present in the thymus tissues of AKR mice prior to the appearance of full-length (8.4-kilobase [kb]) recombinant MCF viral RNAs (A. S. Khan, F. Laigret, and C. P. Rodi, J. Virol. 61:876-882, 1987). These potential MCF env precursors consisted of 7.2-, 3.0-, and 1.8-kb RNA species. In this study, we have determined the structure of the MCF-related mRNAs on the basis of Northern (RNA) blot hybridization analyses by using 10 different MuLV subgenomic DNA probes, determined the nucleotide sequence of a cloned cDNA segment representing the 3' portion of the 7.2-kb mRNA, and studied the expression of ecotropic and xenotropic MuLV sequences by using env-specific DNA probes. The results indicated that ecotropic, xenotropic, and MCF-related transcripts were constitutively and concurrently expressed exclusively in thymus tissue of 2-month-old AKR mice prior to detection of MCF viral RNAs. We have molecularly characterized these thymic MuLV RNAs, which may participate in formation of recombinant MCF viruses; a novel recombinant ecotropic viral RNA was identified as a putative intermediate in the stepwise generation of leukemogenic MCF MuLVs. We have also described the unique structure of the 6.0-kb MCF-related RNAs which were expressed specifically in liver and kidney tissues of AKR mice; these RNAs contained an upstream non-MuLV transcriptional regulatory element.
Full text
PDF

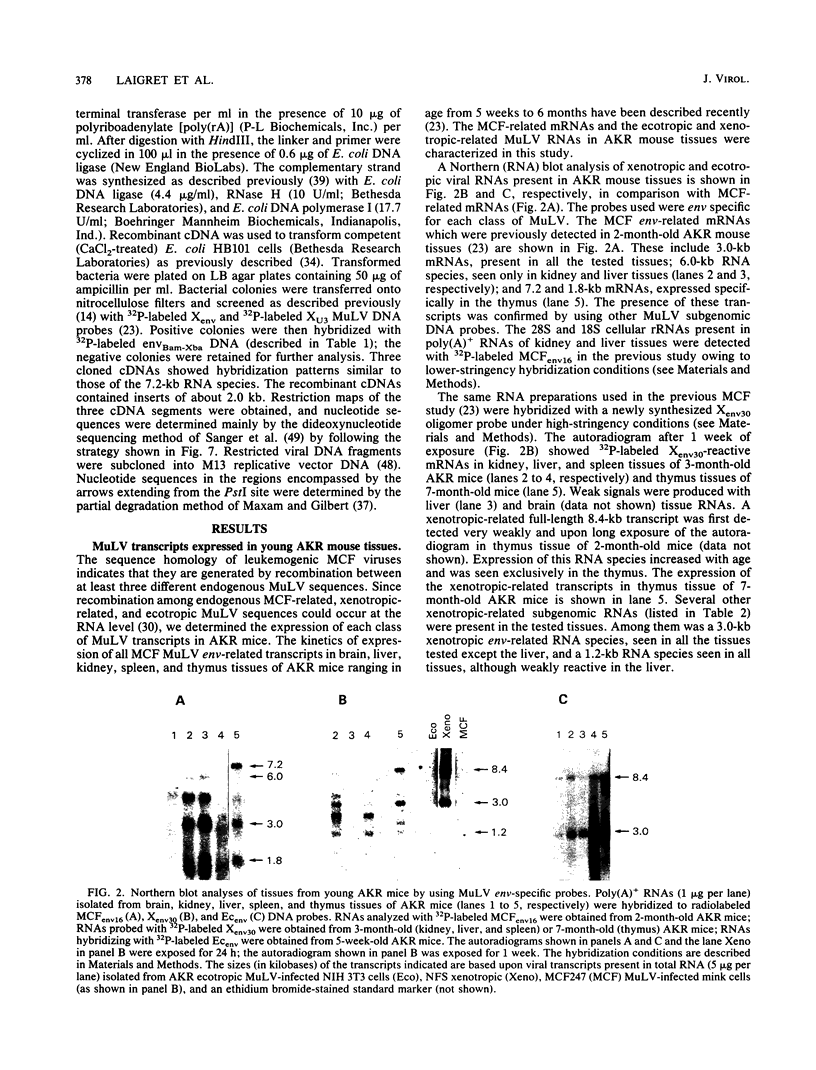
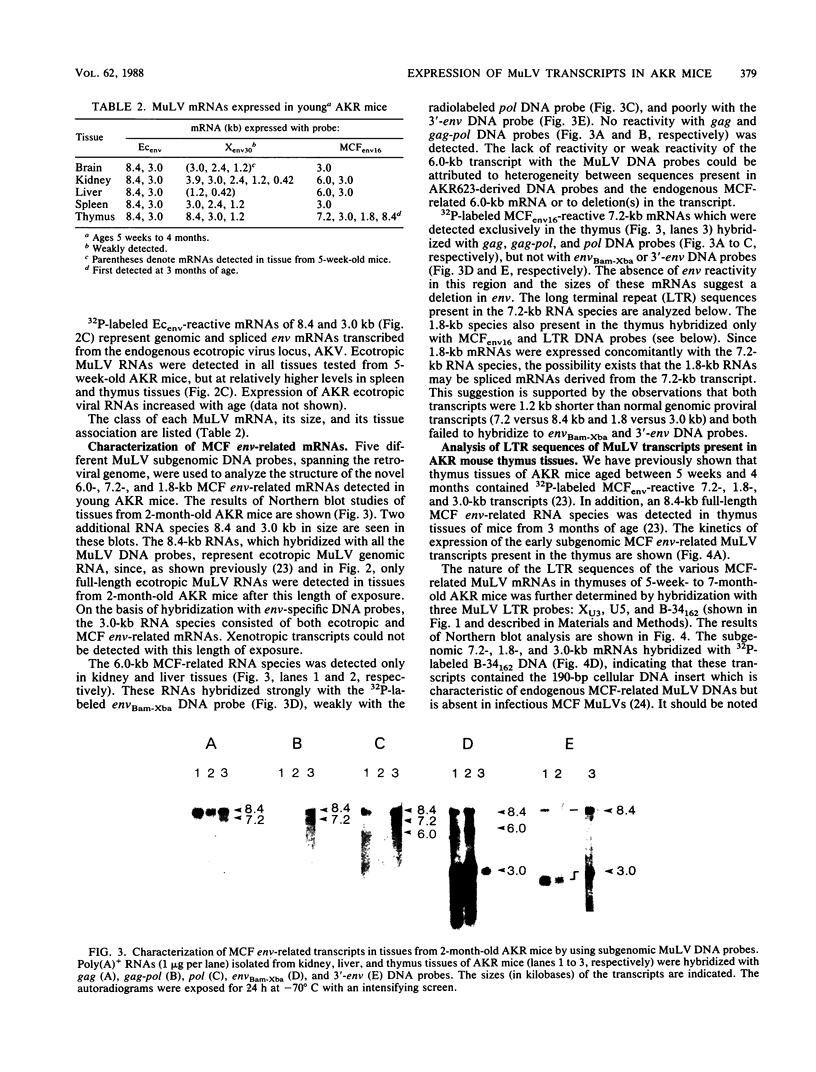
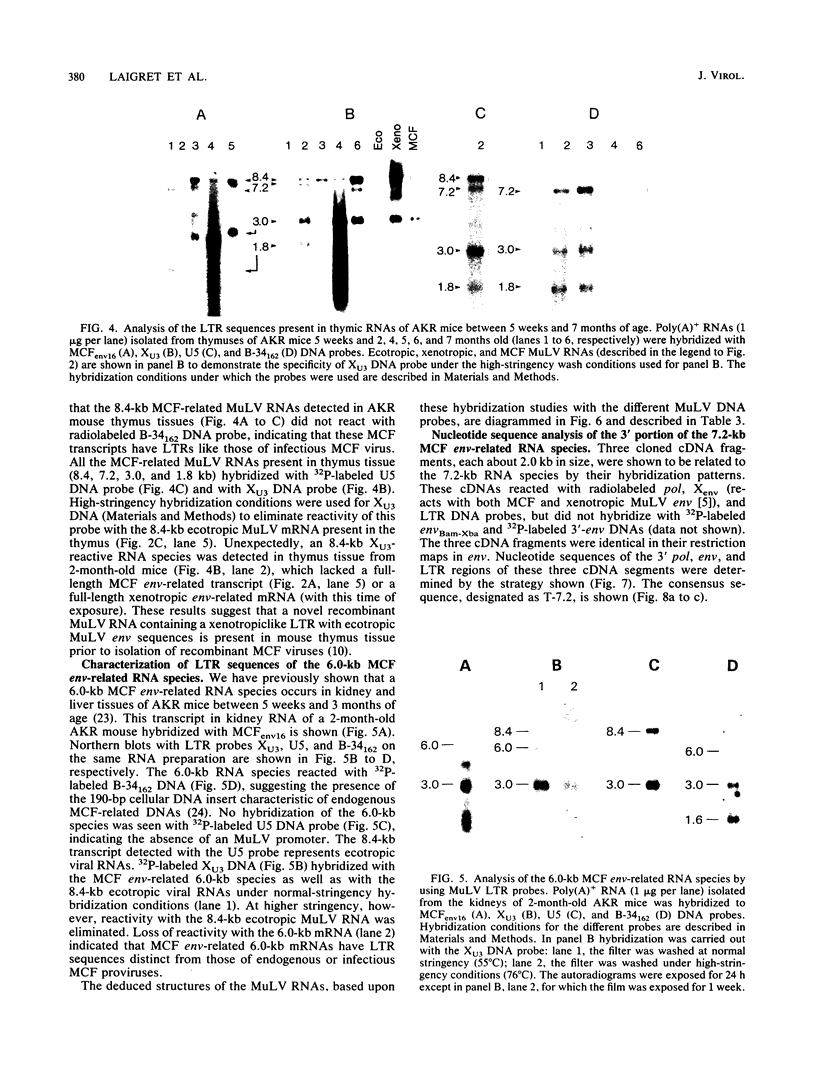
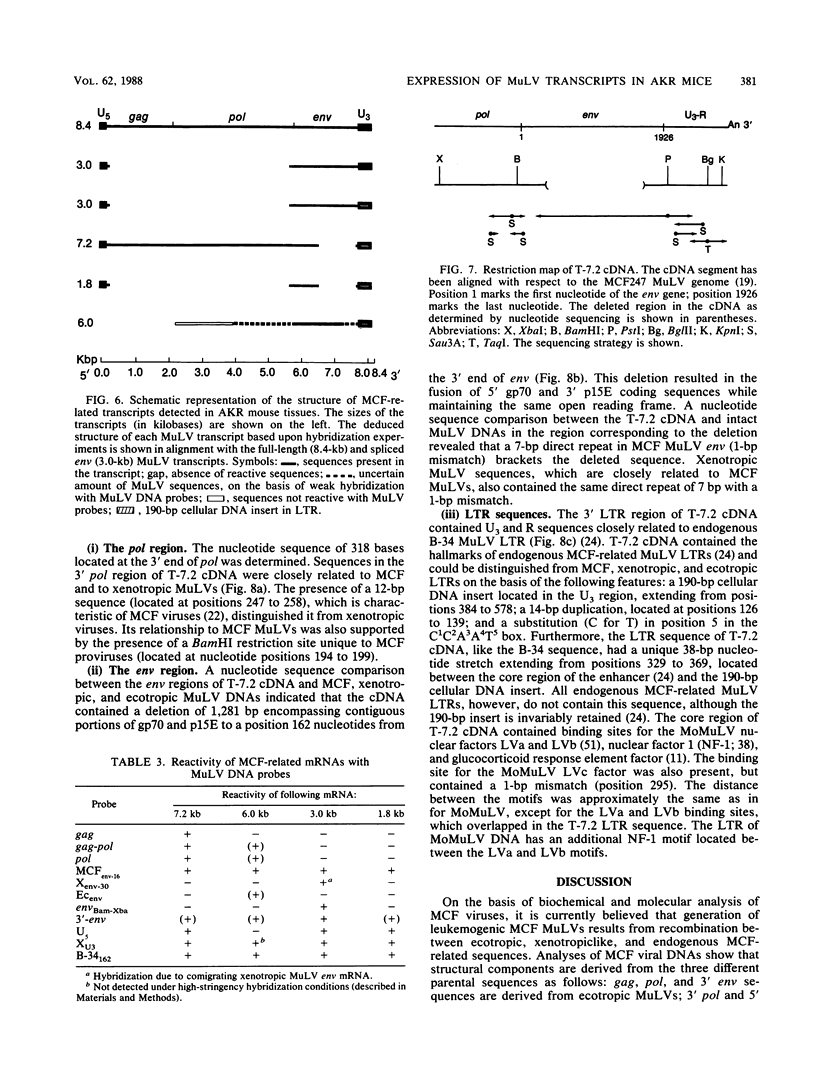





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Sakai K., Kitamura N., Nakanishi S., Niwa O., Matsuyama M., Ishimoto A. Characterization of the env gene and long terminal repeat of molecularly cloned Friend mink cell focus-inducing virus DNA. J Virol. 1984 Jun;50(3):813–821. doi: 10.1128/jvi.50.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Hoggan M. D., Chan H. W., Sears J. F., Khan A. S., Moore J. L., Hartley J. W., Rowe W. P., Martin M. A. Cloning and characterization of an envelope-specific probe from xenotropic murine leukemia proviral DNA. J Virol. 1982 Jan;41(1):228–236. doi: 10.1128/jvi.41.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco D., Yamamoto K. R. Two different factors act separately or together to specify functionally distinct activities at a single transcriptional enhancer. Mol Cell Biol. 1986 Apr;6(4):993–1001. doi: 10.1128/mcb.6.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Buckler C. E., Sears J. F., Rowe W. P., Martin M. A. Organization and stability of endogenous xenotropic murine leukemia virus proviral DNA in mouse genomes. J Virol. 1983 Jan;45(1):473–477. doi: 10.1128/jvi.45.1.473-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Laigret F., Rodi C. P. Expression of mink cell focus-forming murine leukemia virus-related transcripts in AKR mice. J Virol. 1987 Mar;61(3):876–882. doi: 10.1128/jvi.61.3.876-882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Repaske R., Garon C. F., Chan H. W., Rowe W. P., Martin M. A. Characterization of proviruses cloned from mink cell focus-forming virus-infected cellular DNA. J Virol. 1982 Feb;41(2):435–448. doi: 10.1128/jvi.41.2.435-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Rowe W. P., Martin M. A. Cloning of endogenous murine leukemia virus-related sequences from chromosomal DNA of BALB/c and AKR/J mice: identification of an env progenitor of AKR-247 mink cell focus-forming proviral DNA. J Virol. 1982 Nov;44(2):625–636. doi: 10.1128/jvi.44.2.625-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Crowther R., Straceski A., Haseltine W. Nucleotide sequence of the Akv env gene. J Virol. 1982 May;42(2):519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. The Gv-1 locus coordinately regulates the expression of multiple endogenous murine retroviruses. Cell. 1985 May;41(1):289–299. doi: 10.1016/0092-8674(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Envelope gene sequence of two in vitro-generated mink cell focus-forming murine leukemia viruses which contain the entire gp70 sequence of the endogenous nonecotropic parent. J Virol. 1984 Feb;49(2):530–539. doi: 10.1128/jvi.49.2.530-539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Pogue-Geile K., Guntaka R., Staskus K. A., Faras A. J. Involvement of directly repeated sequences in the generation of deletions of the avian sarcoma virus src gene. J Virol. 1983 Aug;47(2):380–382. doi: 10.1128/jvi.47.2.380-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Boone L. R., Yang W. K. A novel sequence segment and other nucleotide structural features in the long terminal repeat of a BALB/c mouse genomic leukemia virus-related DNA clone. Nucleic Acids Res. 1983 Aug 25;11(16):5603–5620. doi: 10.1093/nar/11.16.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Hamagishi Y., Steele P. E., Tykocinski M., Martin M. A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985 Oct;56(1):176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., O'Neill R. R., Khan A. S., Martin M. A. Nucleotide sequence of the env-specific segment of NFS-Th-1 xenotropic murine leukemia virus. J Virol. 1983 Apr;46(1):204–211. doi: 10.1128/jvi.46.1.204-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Young J. M., Mural R. J., Bell T. E., Ihle J. N. Molecular cloning and characterization of murine leukemia virus-related DNA sequences from C3H/HeN mouse DNA. J Virol. 1982 Jul;43(1):113–126. doi: 10.1128/jvi.43.1.113-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]