Abstract
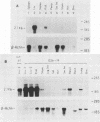
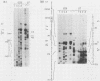
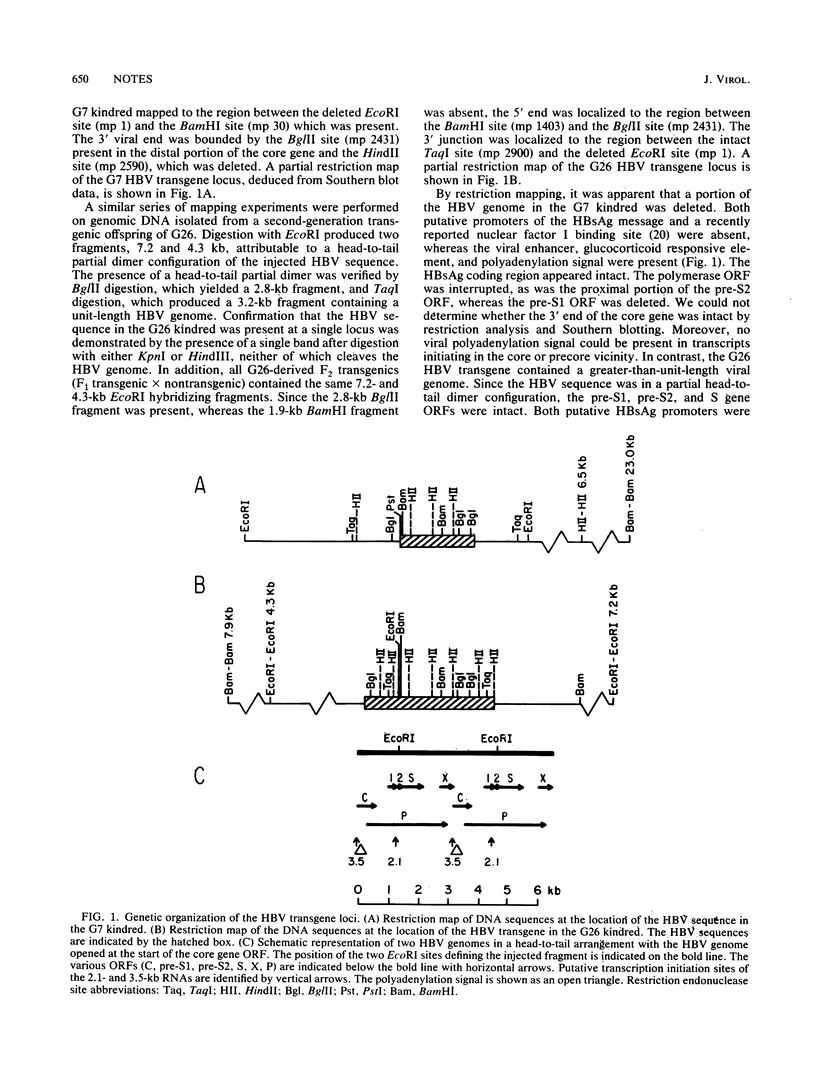
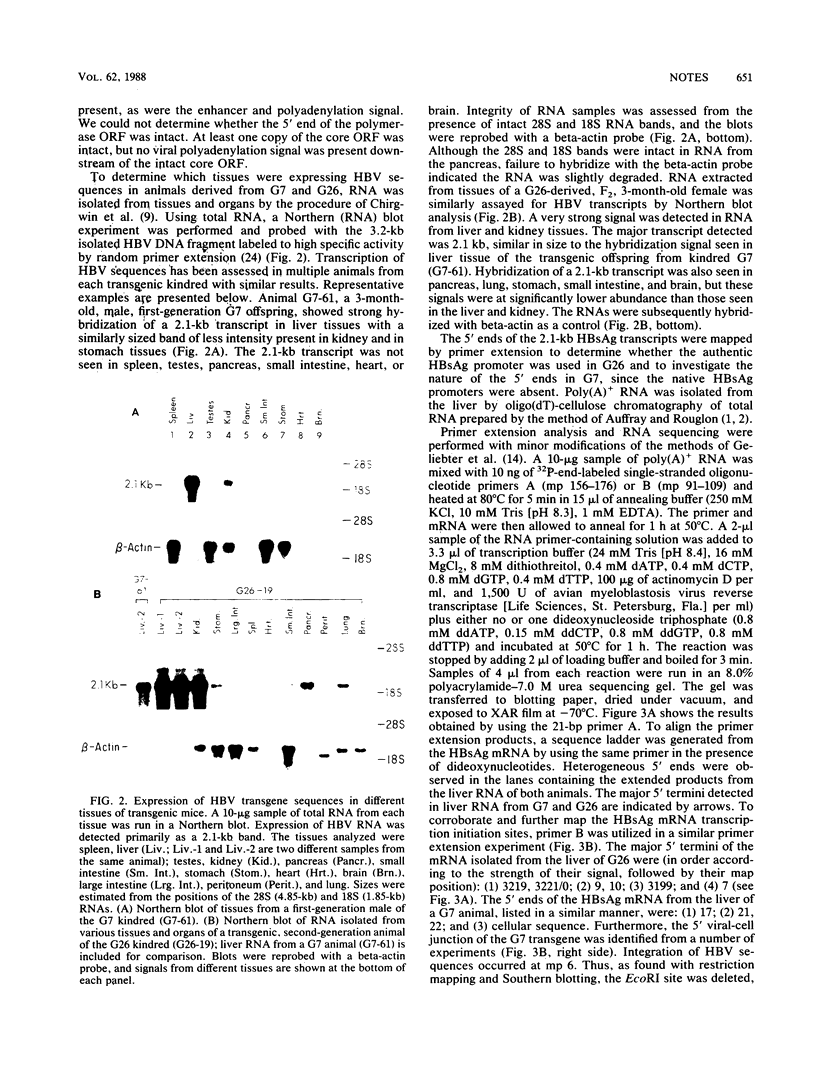
Two transgenic mice were produced by microinjection of the entire hepatitis B virus (HBV) genome as a 3.2-kilobase EcoRI DNA fragment into one-cell embryos. Each animal contained a single, unique locus of HBV sequence. One founder animal, G7, contained a partially deleted HBV genome lacking both putative HBV surface antigen (HBsAg) promoters. The other animal, G26, contained greater-than-genome-length HBV sequences organized as a partial head-to-tail dimer. Both transgenic animals transmitted the HBV sequences in a Mendelian fashion, and all subsequent transgenic animals had detectable HBsAg in the serum. Expression of HBV sequences in tissues from G7- and G26-derived mice showed preferential expression of the 2.1-kilobase HBsAg RNA transcript in liver and kidney tissues by Northern (RNA) blot analysis. These data are consistent with the notion that HBV DNA contains cis-acting regulatory sequences which are responsible for the predominant expression of HBsAg transcripts in the liver and kidney of transgenic mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinet C., Farza H., Morello D., Hadchouel M., Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science. 1985 Dec 6;230(4730):1160–1163. doi: 10.1126/science.3865370. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk R. D., Ma P., Smith K. D. Characterization and evolution of a single-copy sequence from the human Y chromosome. Mol Cell Biol. 1985 Mar;5(3):576–581. doi: 10.1128/mcb.5.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Schaller H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984 Sep;3(9):2191–2196. doi: 10.1002/j.1460-2075.1984.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Pinkert C. A., Milich D. R., Filippi P., McLachlan A., Palmiter R. D., Brinster R. L. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science. 1985 Dec 6;230(4730):1157–1160. doi: 10.1126/science.3865369. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Cummings I. W., Browne J. K., Salser W. A., Tyler G. V., Snyder R. L., Smolec J. M., Summers J. Isolation, characterization, and comparison of recombinant DNAs derived from genomes of human hepatitis B virus and woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1842–1846. doi: 10.1073/pnas.77.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J., Stafford J., Queen C. An immunoglobulin promoter displays cell-type specificity independently of the enhancer. 1985 May 30-Jun 5Nature. 315(6018):423–425. doi: 10.1038/315423a0. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Deery D. T., Petcu D. J., Mason W. S., Molnar-Kimber K. L. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4865–4869. doi: 10.1073/pnas.80.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel S., Siddiqui A. The human hepatitis B virus enhancer requires trans-acting cellular factor(s) for activity. Mol Cell Biol. 1986 Feb;6(2):710–715. doi: 10.1128/mcb.6.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ben-Levy R., De-Medina T. High affinity binding site for nuclear factor I next to the hepatitis B virus S gene promoter. EMBO J. 1986 Aug;5(8):1967–1971. doi: 10.1002/j.1460-2075.1986.tb04451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Jameel S., Mapoles J. Transcriptional control elements of hepatitis B surface antigen gene. Proc Natl Acad Sci U S A. 1986 Feb;83(3):566–570. doi: 10.1073/pnas.83.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Rutter W. J., Varmus H. E., Ganem D. Transcription of the hepatitis B surface antigen gene in cultured murine cells initiates within the presurface region. J Virol. 1984 May;50(2):563–571. doi: 10.1128/jvi.50.2.563-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tognoni A., Cattaneo R., Serfling E., Schaffner W. A novel expression selection approach allows precise mapping of the hepatitis B virus enhancer. Nucleic Acids Res. 1985 Oct 25;13(20):7457–7472. doi: 10.1093/nar/13.20.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M., Laub O. Identification of a promoter element located upstream from the hepatitis B virus X gene. Mol Cell Biol. 1987 Jan;7(1):545–548. doi: 10.1128/mcb.7.1.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Kaspa R., Burk R. D., Shaul Y., Shafritz D. A. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]