Abstract
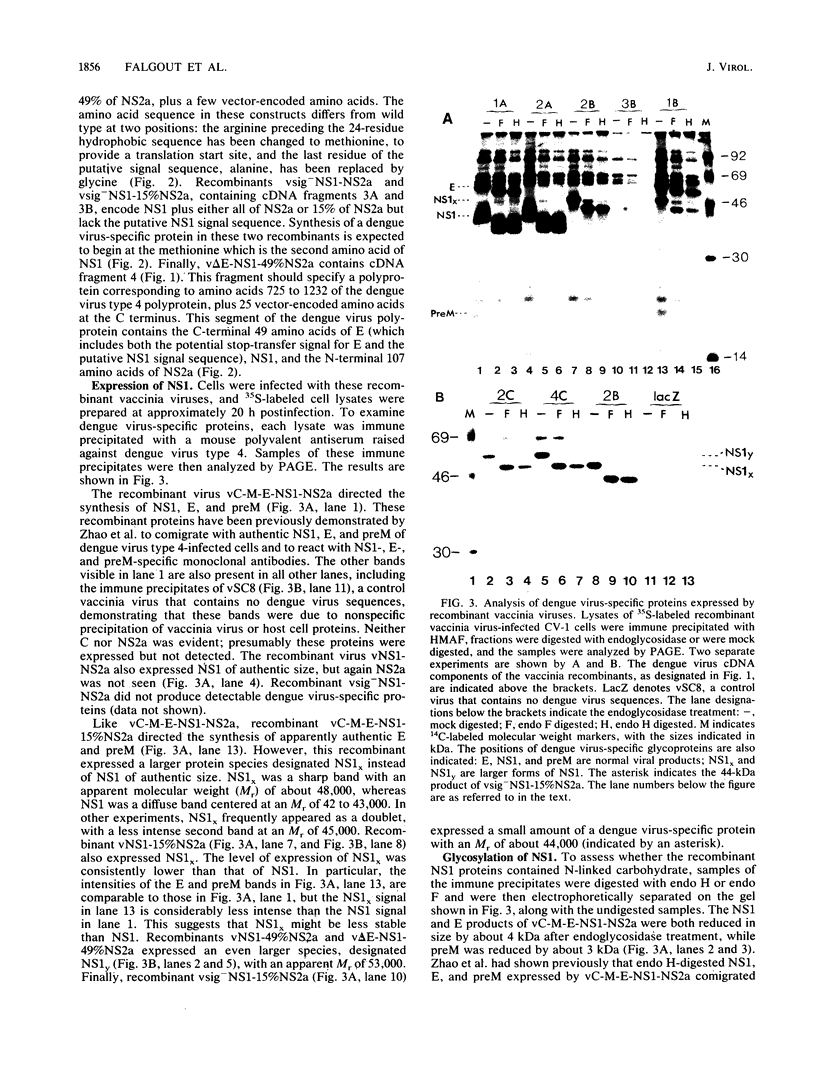
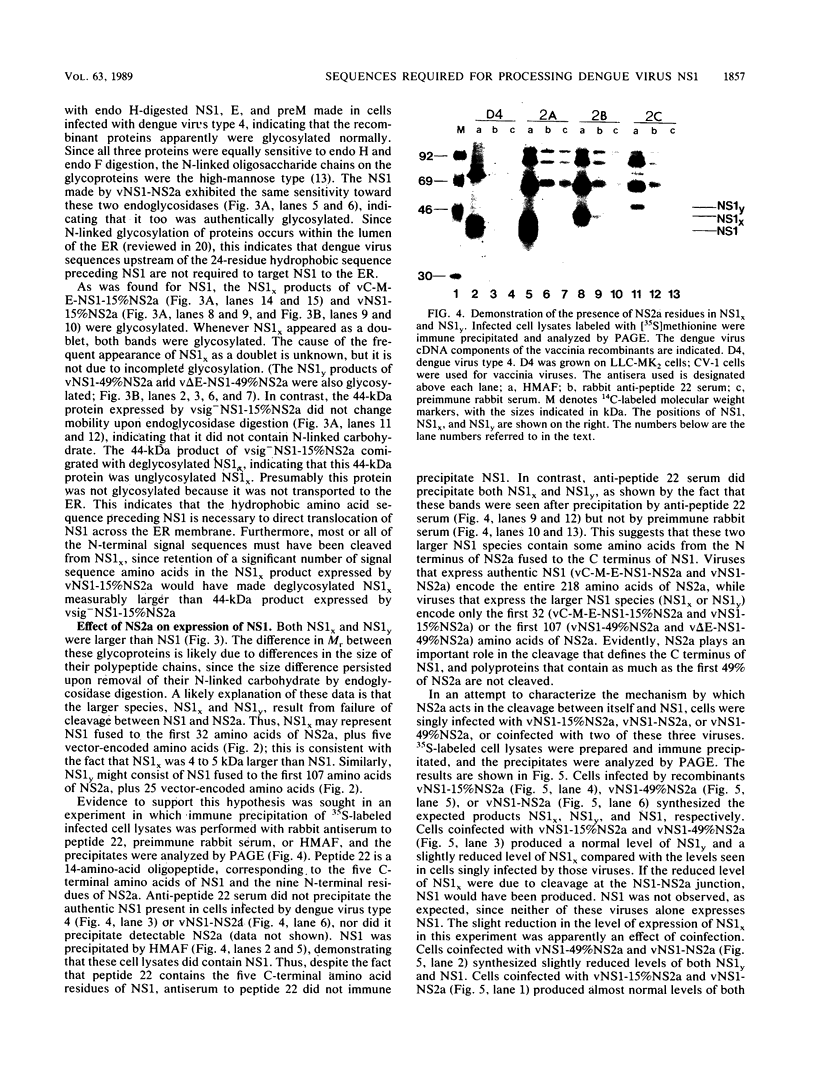
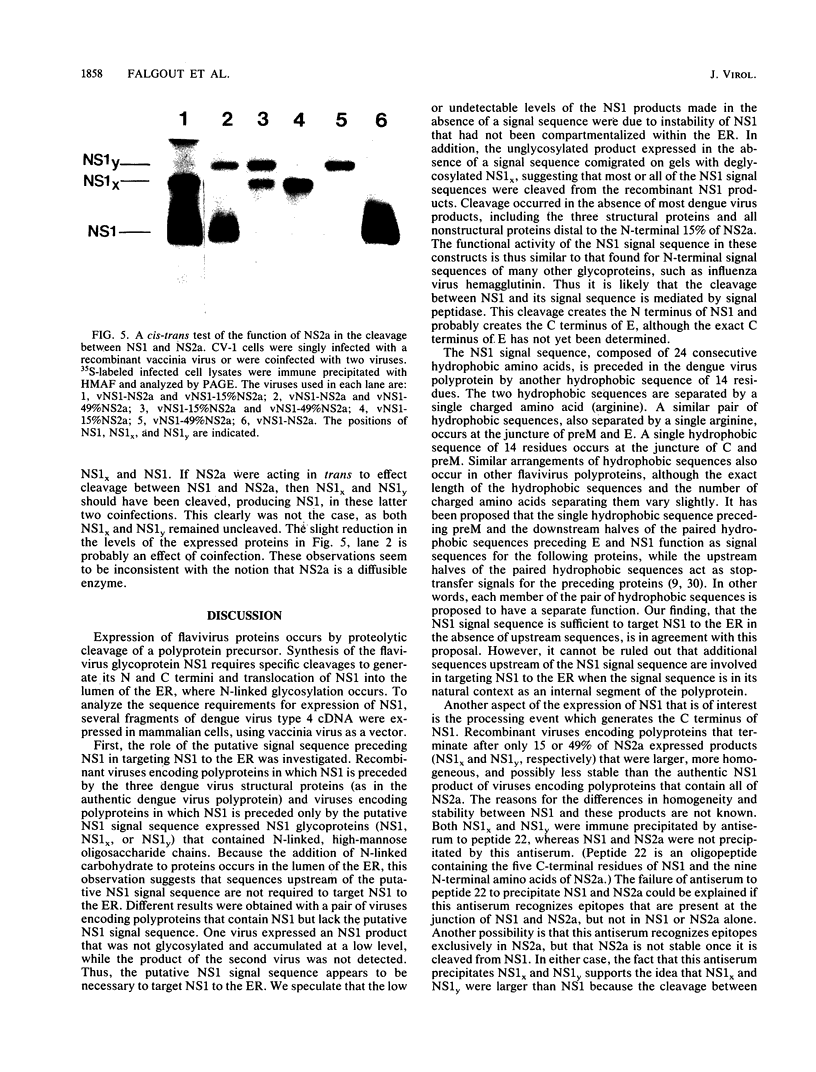
Expression of dengue virus gene products involves specific proteolytic cleavages of a precursor polyprotein. To study the flanking sequences required for expression of the dengue virus nonstructural glycoprotein NS1, we constructed a series of recombinant vaccinia viruses that contain the coding sequence for NS1 in combination with various lengths of upstream and downstream sequences. The NS1 products expressed by these viruses in infected CV-1 cells were immune precipitated and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The data show that the 24-residue hydrophobic sequence preceding NS1 was necessary and sufficient for the production of glycosylated NS1 and that this sequence was cleaved from NS1 in the absence of most dengue virus proteins. This finding is consistent with previous proposals that this hydrophobic sequence serves as an N-terminal signal sequence that is cleaved by signal peptidase. The cleavage between the C terminus of NS1 and the downstream protein NS2a occurred when the complete NS2a was present. Recombinant viruses containing NS1 plus 15 or 49% of NS2a produced proteins larger than authentic NS1, indicating that the cleavage between NS1 and NS2a had not occurred. Failure of cleavage was not corrected by coinfection with a recombinant virus capable of cleavage. These results suggest that NS2a may be a cis-acting protease that cleaves itself from NS1, or NS2a may provide sequences for recognition by a specific cellular protease that cleaves at the NS1-NS2a junction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedrzycka A., Cauchi M. R., Bartholomeusz A., Gorman J. J., Wright P. J. Characterization of protease cleavage sites involved in the formation of the envelope glycoprotein and three non-structural proteins of dengue virus type 2, New Guinea C strain. J Gen Virol. 1987 May;68(Pt 5):1317–1326. doi: 10.1099/0022-1317-68-5-1317. [DOI] [PubMed] [Google Scholar]
- Bonner W. M. Use of fluorography for sensitive isotope detection in polyacrylamide gel electrophoresis and related techniques. Methods Enzymol. 1983;96:215–222. doi: 10.1016/s0076-6879(83)96019-6. [DOI] [PubMed] [Google Scholar]
- Boulton R. W., Westaway E. G. Togavirus RNA: reversible effect of urea on genomes and absence of subgenomic viral RNA in Kunjin virus-infected cells. Arch Virol. 1977;55(3):201–208. doi: 10.1007/BF01319906. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Lund J. K. Distribution of dengue-2 antigens by electron immunocytochemistry. Infect Immun. 1976 Jun;13(6):1699–1709. doi: 10.1128/iai.13.6.1699-1709.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E., Leidner U., Nowak T., Wengler G., Wengler G. Primary structure of the West Nile flavivirus genome region coding for all nonstructural proteins. Virology. 1986 Feb;149(1):10–26. doi: 10.1016/0042-6822(86)90082-6. [DOI] [PubMed] [Google Scholar]
- Castle E., Nowak T., Leidner U., Wengler G., Wengler G. Sequence analysis of the viral core protein and the membrane-associated proteins V1 and NV2 of the flavivirus West Nile virus and of the genome sequence for these proteins. Virology. 1985 Sep;145(2):227–236. doi: 10.1016/0042-6822(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaves G. R., Dubin D. T. Methylation status of intracellular dengue type 2 40 S RNA. Virology. 1979 Jul 15;96(1):159–165. doi: 10.1016/0042-6822(79)90181-8. [DOI] [PubMed] [Google Scholar]
- Coia G., Parker M. D., Speight G., Byrne M. E., Westaway E. G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988 Jan;69(Pt 1):1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Trent D. W., Strauss J. H., Rice C. M. Partial nucleotide sequence of the Murray Valley encephalitis virus genome. Comparison of the encoded polypeptides with yellow fever virus structural and non-structural proteins. J Mol Biol. 1986 Feb 5;187(3):309–323. doi: 10.1016/0022-2836(86)90435-3. [DOI] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Trent D. W. Nucleotide sequence and deduced amino acid sequence of the nonstructural proteins of dengue type 2 virus, Jamaica genotype: comparative analysis of the full-length genome. Virology. 1988 Jul;165(1):234–244. doi: 10.1016/0042-6822(88)90677-0. [DOI] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Trent D. W. Nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue type 2 virus, Jamaica genotype. Virology. 1986 Dec;155(2):365–377. doi: 10.1016/0042-6822(86)90200-x. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Henchal E. A., Repik P. M., McCown J. M., Brandt W. E. Identification of an antigenic and genetic variant of dengue-4 virus from the Caribbean. Am J Trop Med Hyg. 1986 Mar;35(2):393–400. doi: 10.4269/ajtmh.1986.35.393. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Trent D. W. Identification of Saint Louis encephalitis virus mRNA. J Virol. 1978 Feb;25(2):535–545. doi: 10.1128/jvi.25.2.535-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Aebersold R., Teplow D. B., Pata J., Bell J. R., Vorndam A. V., Trent D. W., Brandriss M. W., Schlesinger J. J., Strauss J. H. Partial N-terminal amino acid sequences of three nonstructural proteins of two flaviviruses. Virology. 1986 May;151(1):1–9. doi: 10.1016/0042-6822(86)90098-x. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Cropp C. B., Monath T. P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986 Dec;60(3):1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985 Oct;135(4):2805–2809. [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol. 1987 Mar;68(Pt 3):853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Wright P. J. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected vero and Aedes albopictus cells. J Gen Virol. 1985 Mar;66(Pt 3):559–571. doi: 10.1099/0022-1317-66-3-559. [DOI] [PubMed] [Google Scholar]
- Speight G., Coia G., Parker M. D., Westaway E. G. Gene mapping and positive identification of the non-structural proteins NS2A, NS2B, NS3, NS4B and NS5 of the flavivirus Kunjin and their cleavage sites. J Gen Virol. 1988 Jan;69(Pt 1):23–34. doi: 10.1099/0022-1317-69-1-23. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi H., Mori C., Fuke I., Morita K., Kuhara S., Kondou J., Kikuchi Y., Nagamatu H., Igarashi A. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology. 1987 Dec;161(2):497–510. doi: 10.1016/0042-6822(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Kinney R. M., Johnson B. J., Vorndam A. V., Grant J. A., Deubel V., Rice C. M., Hahn C. Partial nucleotide sequence of St. Louis encephalitis virus RNA: structural proteins, NS1, ns2a, and ns2b. Virology. 1987 Feb;156(2):293–304. doi: 10.1016/0042-6822(87)90409-0. [DOI] [PubMed] [Google Scholar]
- Vijaya S., Elango N., Zavala F., Moss B. Transport to the cell surface of a peptide sequence attached to the truncated C terminus of an N-terminally anchored integral membrane protein. Mol Cell Biol. 1988 Apr;8(4):1709–1714. doi: 10.1128/mcb.8.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Castle E., Leidner U., Nowak T., Wengler G. Sequence analysis of the membrane protein V3 of the flavivirus West Nile virus and of its gene. Virology. 1985 Dec;147(2):264–274. doi: 10.1016/0042-6822(85)90129-1. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Flaviviridae. Intervirology. 1985;24(4):183–192. doi: 10.1159/000149642. [DOI] [PubMed] [Google Scholar]
- Zhao B. T., Prince G., Horswood R., Eckels K., Summers P., Chanock R., Lai C. J. Expression of dengue virus structural proteins and nonstructural protein NS1 by a recombinant vaccinia virus. J Virol. 1987 Dec;61(12):4019–4022. doi: 10.1128/jvi.61.12.4019-4022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Mackow E., Buckler-White A., Markoff L., Chanock R. M., Lai C. J., Makino Y. Cloning full-length dengue type 4 viral DNA sequences: analysis of genes coding for structural proteins. Virology. 1986 Nov;155(1):77–88. doi: 10.1016/0042-6822(86)90169-8. [DOI] [PubMed] [Google Scholar]