Abstract
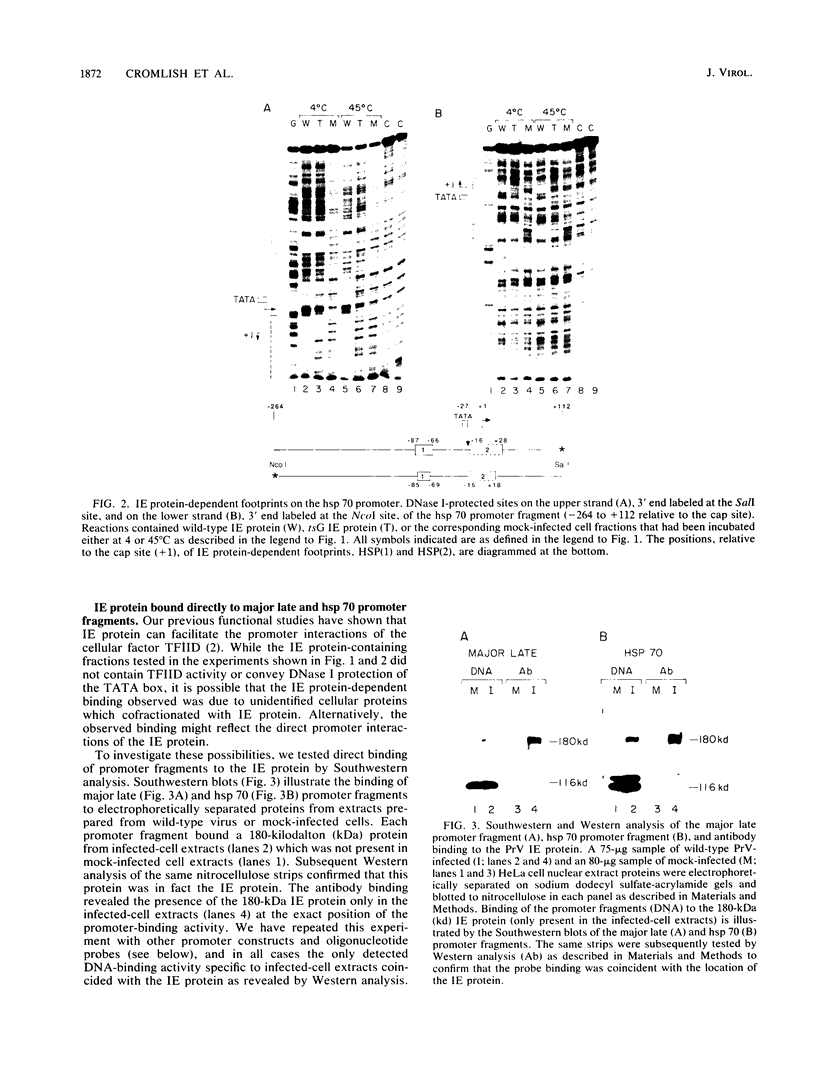
In the presence of partially purified pseudorabies virus immediate-early protein, multiple sites of DNase I protection were observed on the adenovirus major late and human hsp 70 promoters. Southwestern (DNA-protein blot) analysis demonstrated that the immediate-early protein bound directly to the sequences contained in these sites. These sequences share only limited homology, differ in their affinities for the immediate-early protein, and are located at different positions on these two promoters. In addition, the site-specific binding of a temperature-sensitive immediate-early protein was eliminated by the same heat treatment which eliminates its transcriptional activating function, whereas the binding of the wild-type protein was unaffected by heat treatment. Thus, site-specific binding requires a functionally active immediate-early protein. Furthermore, immediate-early-protein-dependent in vitro transcription from the major late promoter was preferentially inhibited by oligonucleotides which are homologous to the high-affinity binding sites on the major late or hsp 70 promoters. These observations suggest that transcriptional stimulation by the immediate-early protein involves binding to cis-acting elements.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abmayr S. M., Feldman L. D., Roeder R. G. In vitro stimulation of specific RNA polymerase II-mediated transcription by the pseudorabies virus immediate early protein. Cell. 1985 Dec;43(3 Pt 2):821–829. doi: 10.1016/0092-8674(85)90255-7. [DOI] [PubMed] [Google Scholar]
- Abmayr S. M., Workman J. L., Roeder R. G. The pseudorabies immediate early protein stimulates in vitro transcription by facilitating TFIID: promoter interactions. Genes Dev. 1988 May;2(5):542–553. doi: 10.1101/gad.2.5.542. [DOI] [PubMed] [Google Scholar]
- Ahlers S. E., Feldman L. T. Effects of a temperature-sensitive mutation in the immediate-early gene of pseudorabies virus on class II and class III gene transcription. J Virol. 1987 Apr;61(4):1103–1107. doi: 10.1128/jvi.61.4.1103-1107.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers S. E., Feldman L. T. Immediate-early protein of pseudorabies virus is not continuously required to reinitiate transcription of induced genes. J Virol. 1987 Apr;61(4):1258–1260. doi: 10.1128/jvi.61.4.1258-1260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Bruner M., Flint S. J., Harter M. L. DNA-binding properties of an adenovirus 289R E1A protein. EMBO J. 1988 Mar;7(3):835–841. doi: 10.1002/j.1460-2075.1988.tb02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlan C. A., Coulter C., Feldman L. T. Binding of the pseudorabies virus immediate-early protein to single-stranded DNA. J Virol. 1987 Jun;61(6):1855–1860. doi: 10.1128/jvi.61.6.1855-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Weinheimer S. P., McKnight S. L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986 Oct 3;234(4772):53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of herpes simplex virus regulatory protein ICP4 with sequences spanning the ICP4 gene transcription initiation site. Nucleic Acids Res. 1988 Jan 25;16(2):555–570. doi: 10.1093/nar/16.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber S. W., Wilcox K. W. Association of the herpes simplex virus regulatory protein ICP4 with specific nucleotide sequences in DNA. Nucleic Acids Res. 1986 Aug 11;14(15):6067–6083. doi: 10.1093/nar/14.15.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Ihara S., Feldman L., Watanabe S., Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983 Dec;131(2):437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kristie T. M., Roizman B. Alpha 4, the major regulatory protein of herpes simplex virus type 1, is stably and specifically associated with promoter-regulatory domains of alpha genes and of selected other viral genes. Proc Natl Acad Sci U S A. 1986 May;83(10):3218–3222. doi: 10.1073/pnas.83.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., Roeder R. G. The use of monoclonal antibodies for the characterization of a 5 S gene-specific transcription factor (IIIA) from Xenopus laevis. J Biol Chem. 1983 Oct 10;258(19):11915–11923. [PubMed] [Google Scholar]
- Lee K. A., Hai T. Y., SivaRaman L., Thimmappaya B., Hurst H. C., Jones N. C., Green M. R. A cellular protein, activating transcription factor, activates transcription of multiple E1A-inducible adenovirus early promoters. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8355–8359. doi: 10.1073/pnas.84.23.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K., Brunet L., Berk A. J. Factors responsible for the higher transcriptional activity of extracts of adenovirus-infected cells fractionate with the TATA box transcription factor. Mol Cell Biol. 1988 Apr;8(4):1765–1774. doi: 10.1128/mcb.8.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Michael N., Spector D., Mavromara-Nazos P., Kristie T. M., Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988 Mar 25;239(4847):1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Spangler R., Bruner M., Dalie B., Harter M. L. Activation of adenovirus promoters by the adenovirus E1A protein in cell-free extracts. Science. 1987 Aug 28;237(4818):1044–1046. doi: 10.1126/science.2956686. [DOI] [PubMed] [Google Scholar]
- Tedder D. G., Pizer L. I. Role for DNA-protein interaction in activation of the herpes simplex virus glycoprotein D gene. J Virol. 1988 Dec;62(12):4661–4672. doi: 10.1128/jvi.62.12.4661-4672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M. L., Yee S. P., Persson R. H., Bacchetti S., Smiley J. R., Branton P. E. Activation and inhibition of expression of the 72,000-Da early protein of adenovirus type 5 in mouse cells constitutively expressing an immediate early protein of herpes simplex virus type 1. Virology. 1985 Jul 15;144(1):35–45. doi: 10.1016/0042-6822(85)90302-2. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Roeder R. G., Sawadogo M. Physical analysis of transcription preinitiation complex assembly on a class II gene promoter. Science. 1988 Sep 9;241(4871):1335–1338. doi: 10.1126/science.3413495. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Abmayr S. M., Cromlish W. A., Roeder R. G. Transcriptional regulation by the immediate early protein of pseudorabies virus during in vitro nucleosome assembly. Cell. 1988 Oct 21;55(2):211–219. doi: 10.1016/0092-8674(88)90044-x. [DOI] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]