Abstract
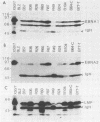
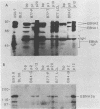
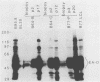
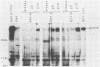
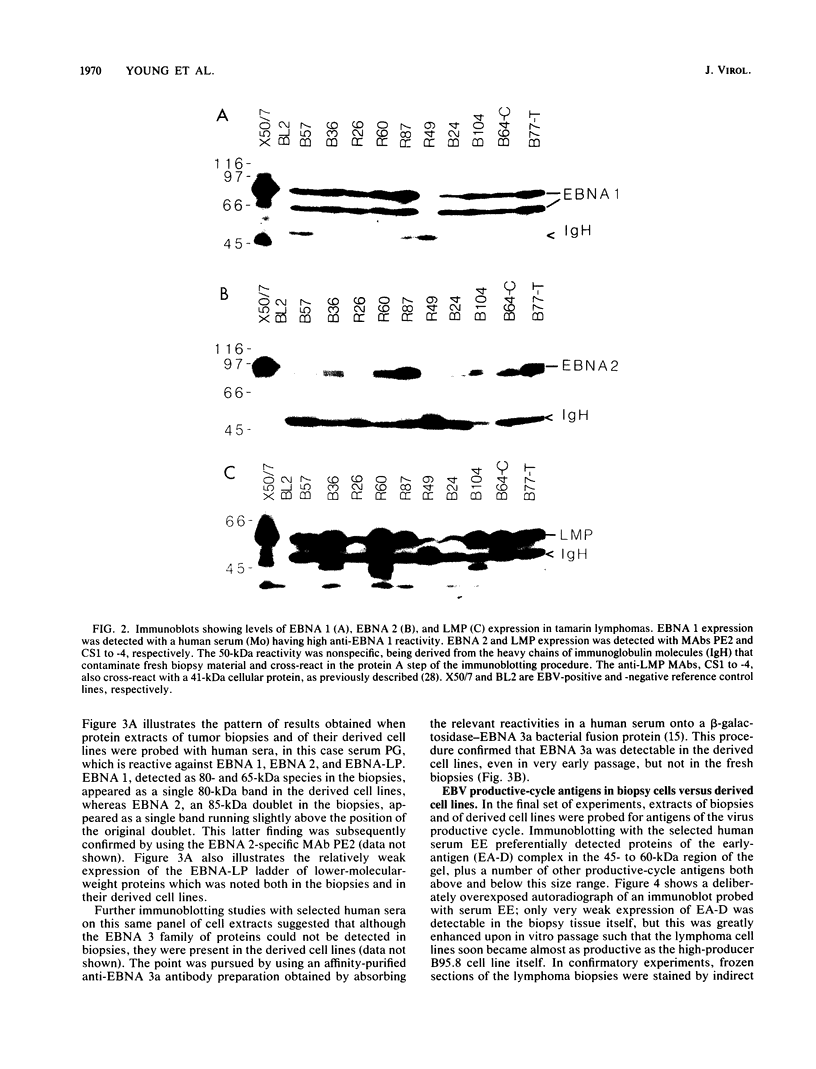
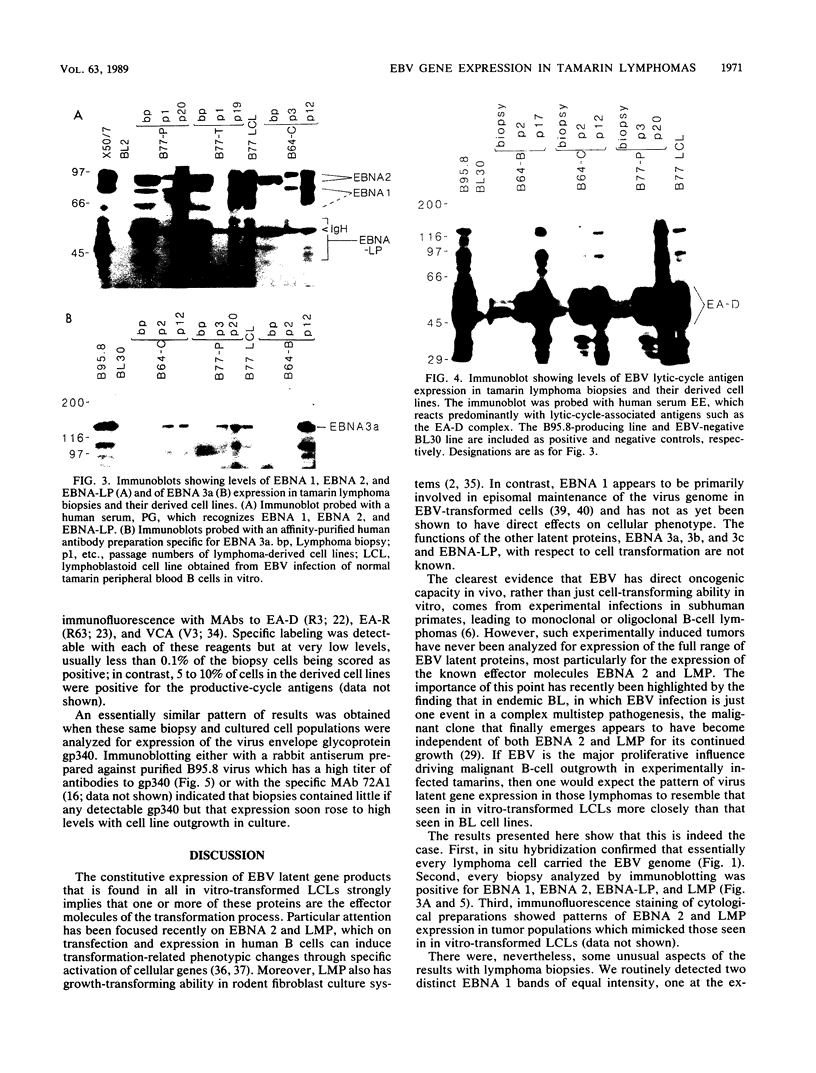
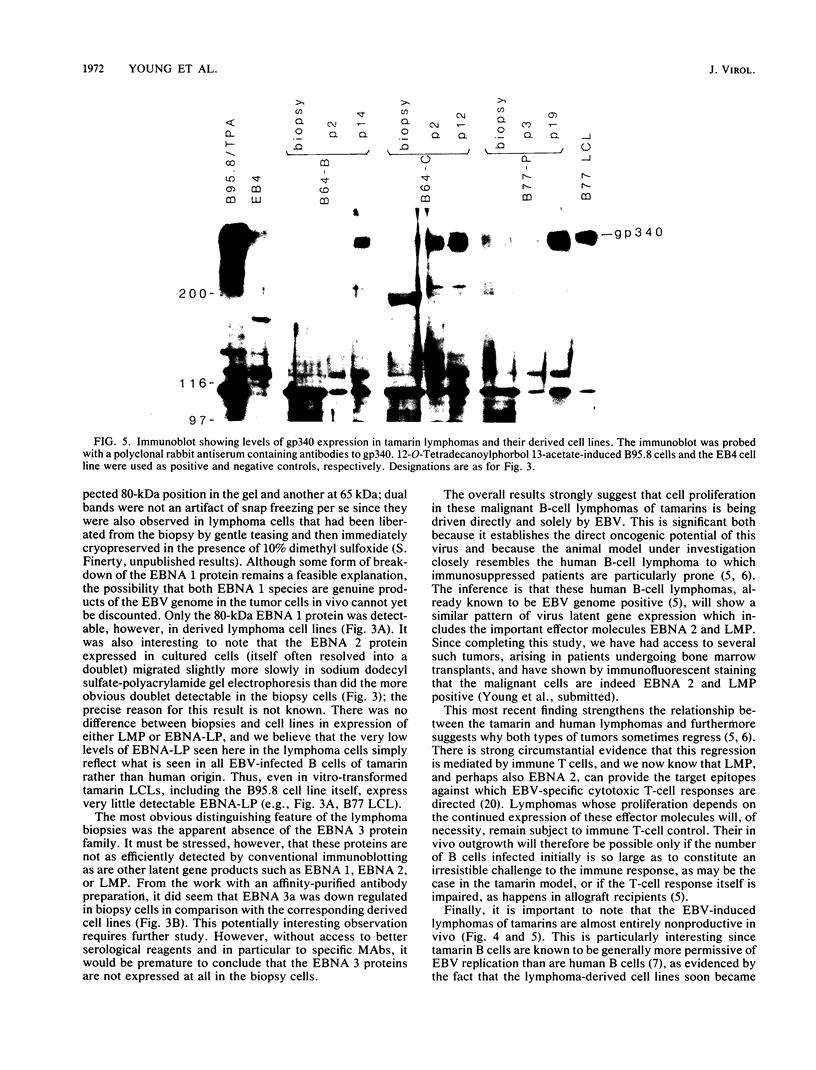
Inoculation of cottontop tamarins with a large dose of Epstein-Barr virus (EBV) leads to the induction of multiple EBV genome-positive lymphomas. These tumors have been characterized as oligoclonal or monoclonal large-cell malignant lymphomas that closely resemble the EBV genome-positive B-cell lymphomas that arise in human allograft recipients. The expression of latent and lytic EBV-encoded proteins was investigated in these virus-induced tamarin lymphomas and in derived cell lines. The tamarin tumors were found to express EBV nuclear antigen 1 (EBNA 1), EBNA 2, EBNA leader protein, and the latent membrane protein (LMP) as determined both by immunohistochemical staining and by immunoblotting. However, within the limits of the immunoblotting assays, no expression of the EBNA 3a protein family could be detected. Assays for lytic-cycle proteins by using both polyclonal human sera and monoclonal antibodies against viral capsid antigen, early antigen, and membrane antigen (gp340/220) showed minimal, if any, expression of these antigens in the lymphoma biopsies. In contrast, the cell lines derived from these lymphomas, even in early passage, expressed abundant levels of the lytic-cycle antigens and also expressed the EBNA 3a protein as well as EBNA 1, EBNA 2, EBNA leader protein, and LMP. This finding suggests that the virus-lymphoma cell interaction, in particular the switch to lytic cycle, is subject to some form of host control in vivo. The expression of EBNA 2 and LMP in these tamarin lymphomas strengthens their resemblance to posttransplant lymphomas in humans, since these human tumors are also EBNA 2 and LMP positive (L. S. Young, C. Alfieri, K. Hennessy, H. Evans, C. O'Hara, K. Anderson, A. Rickinson, E. Kieff, and J. I. Cohen, submitted for publication). Since both proteins are known to be important effector molecules of virus-induced B-cell growth transformation in vitro, their expression in these lymphomas constitutes the best evidence for a direct oncogenic role for EBV in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambinder R. F., Wingard J. R., Burns W. H., Hayward S. D., Saral R., Perry H. R., Santos G. W., Hayward G. S. Detection of Epstein-Barr virus DNA in mouthwashes by hybridization. J Clin Microbiol. 1985 Mar;21(3):353–356. doi: 10.1128/jcm.21.3.353-356.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baichwal V. R., Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988 May;2(5):461–467. [PubMed] [Google Scholar]
- Burns J., Chan V. T., Jonasson J. A., Fleming K. A., Taylor S., McGee J. O. Sensitive system for visualising biotinylated DNA probes hybridised in situ: rapid sex determination of intact cells. J Clin Pathol. 1985 Oct;38(10):1085–1092. doi: 10.1136/jcp.38.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Epstein M. A., Finerty S., Dorfman R. F., Bornkamm G. W., Kirkwood J. K., Morgan A. J., Sklar J. Individual tumors of multifocal EB virus-induced malignant lymphomas in tamarins arise from different B-cell clones. Science. 1985 May 10;228(4700):722–724. doi: 10.1126/science.2986287. [DOI] [PubMed] [Google Scholar]
- Crawford D. H., Epstein M. A., Bornkamm G. W., Achong B. G., Finerty S., Thompson J. L. Biological and biochemical observations on isolates of EB virus from the malignant epithelial cells of two nasopharyngeal carcinomas. Int J Cancer. 1979 Sep 15;24(3):294–302. doi: 10.1002/ijc.2910240305. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Morgan A. J., Finerty S., Randle B. J., Kirkwood J. K. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature. 1985 Nov 21;318(6043):287–289. doi: 10.1038/318287a0. [DOI] [PubMed] [Google Scholar]
- Finerty S., Scullion F. T., Morgan A. J. Demonstration in vitro of cell mediated immunity to Epstein-Barr virus in cotton-top tamarins. Clin Exp Immunol. 1988 Aug;73(2):181–185. [PMC free article] [PubMed] [Google Scholar]
- Finke J., Rowe M., Kallin B., Ernberg I., Rosén A., Dillner J., Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987 Dec;61(12):3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Fennewald S., Hummel M., Cole T., Kieff E. A membrane protein encoded by Epstein-Barr virus in latent growth-transforming infection. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7207–7211. doi: 10.1073/pnas.81.22.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. A second nuclear protein is encoded by Epstein-Barr virus in latent infection. Science. 1985 Mar 8;227(4691):1238–1240. doi: 10.1126/science.2983420. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Wang F., Bushman E. W., Kieff E. Definitive identification of a member of the Epstein-Barr virus nuclear protein 3 family. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman G. J., Lazarowitz S. G., Hayward S. D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980 May;77(5):2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood J. K., Epstein M. A., Terlecki A. J., Underwood S. J. Rearing a second generation of cotton-top tamarins (Saguinus oedipus oedipus) in captivity. Lab Anim. 1985 Oct;19(4):269–272. doi: 10.1258/002367785780887491. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan A. J., Finerty S., Lovgren K., Scullion F. T., Morein B. Prevention of Epstein-Barr (EB) virus-induced lymphoma in cottontop tamarins by vaccination with the EB virus envelope glycoprotein gp340 incorporated into immune-stimulating complexes. J Gen Virol. 1988 Aug;69(Pt 8):2093–2096. doi: 10.1099/0022-1317-69-8-2093. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Young L. S., Calender A., Gregory C. D., Rowe M., Lenoir G. M., Rickinson A. B. Different patterns of Epstein-Barr virus gene expression and of cytotoxic T-cell recognition in B-cell lines infected with transforming (B95.8) or nontransforming (P3HR1) virus strains. J Virol. 1988 Mar;62(3):894–901. doi: 10.1128/jvi.62.3.894-901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North J. R., Morgan A. J., Epstein M. A. Observations on the EB virus envelope and virus-determined membrane antigen (MA) polypeptides. Int J Cancer. 1980 Aug;26(2):231–240. doi: 10.1002/ijc.2910260216. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Vroman B., Chase B., Sculley T., Hummel M., Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983 Jul;47(1):193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Kieff E. A sixth Epstein-Barr virus nuclear protein (EBNA3B) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Jun;62(6):2173–2178. doi: 10.1128/jvi.62.6.2173-2178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Sample J., Wang F., Kieff E. A fifth Epstein-Barr virus nuclear protein (EBNA3C) is expressed in latently infected growth-transformed lymphocytes. J Virol. 1988 Apr;62(4):1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Young L. S., Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987 May;61(5):1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Evans H. S., Young L. S., Hennessy K., Kieff E., Rickinson A. B. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J Gen Virol. 1987 Jun;68(Pt 6):1575–1586. doi: 10.1099/0022-1317-68-6-1575. [DOI] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Cadwallader K., Petti L., Kieff E., Rickinson A. B. Distinction between Epstein-Barr virus type A (EBNA 2A) and type B (EBNA 2B) isolates extends to the EBNA 3 family of nuclear proteins. J Virol. 1989 Mar;63(3):1031–1039. doi: 10.1128/jvi.63.3.1031-1039.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Yamaki M., Sakuma S., Ono Y., Takada K. Three Epstein-Barr virus (EBV)-determined nuclear antigens induced by the BamHI E region of EBV DNA. Int J Cancer. 1988 May 15;41(5):744–751. doi: 10.1002/ijc.2910410518. [DOI] [PubMed] [Google Scholar]
- Speck S. H., Pfitzner A., Strominger J. L. An Epstein-Barr virus transcript from a latently infected, growth-transformed B-cell line encodes a highly repetitive polypeptide. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9298–9302. doi: 10.1073/pnas.83.24.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaeto D., Wallace L., Morgan A., Morein B., Rickinson A. B. In vitro T cell responses to a candidate Epstein-Barr virus vaccine: human CD4+ T cell clones specific for the major envelope glycoprotein gp340. Eur J Immunol. 1988 Nov;18(11):1689–1697. doi: 10.1002/eji.1830181106. [DOI] [PubMed] [Google Scholar]
- Vroman B., Luka J., Rodriguez M., Pearson G. R. Characterization of a major protein with a molecular weight of 160,000 associated with the viral capsid of Epstein-Barr virus. J Virol. 1985 Jan;53(1):107–113. doi: 10.1128/jvi.53.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988 Nov;62(11):4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Petti L., Braun D., Seung S., Kieff E. A bicistronic Epstein-Barr virus mRNA encodes two nuclear proteins in latently infected, growth-transformed lymphocytes. J Virol. 1987 Apr;61(4):945–954. doi: 10.1128/jvi.61.4.945-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- Young L. S., Yao Q. Y., Rooney C. M., Sculley T. B., Moss D. J., Rupani H., Laux G., Bornkamm G. W., Rickinson A. B. New type B isolates of Epstein-Barr virus from Burkitt's lymphoma and from normal individuals in endemic areas. J Gen Virol. 1987 Nov;68(Pt 11):2853–2862. doi: 10.1099/0022-1317-68-11-2853. [DOI] [PubMed] [Google Scholar]