Abstract
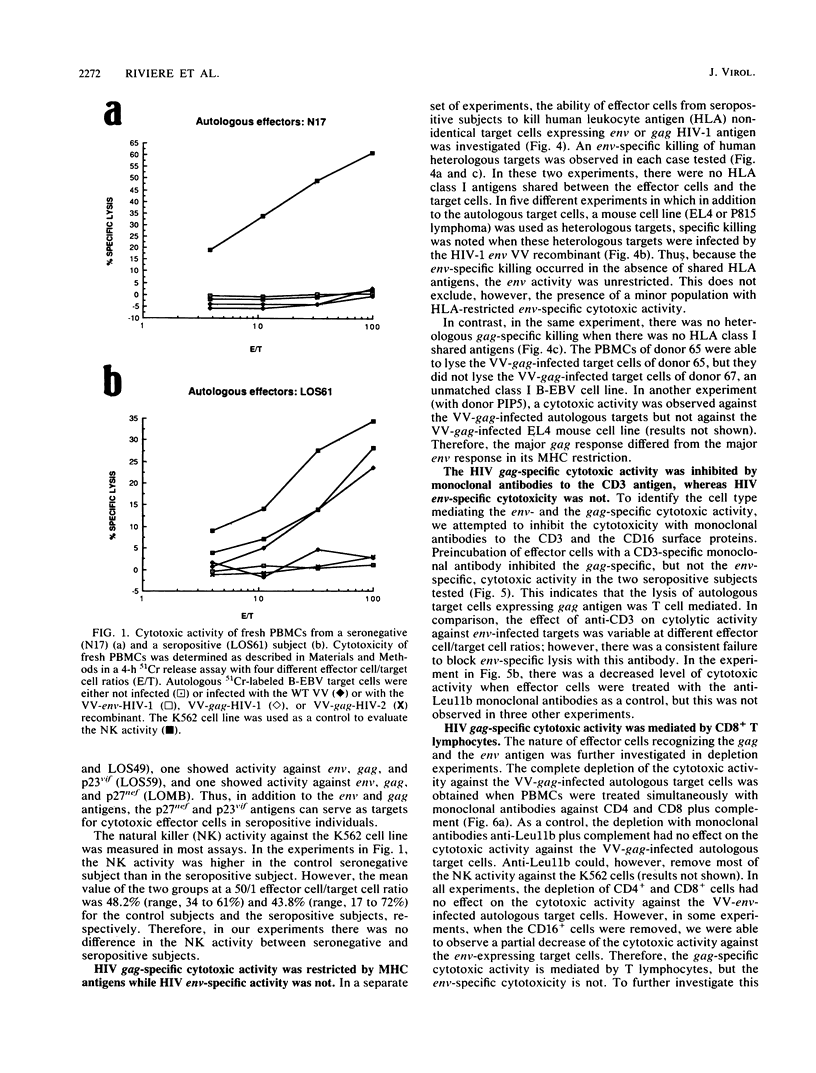
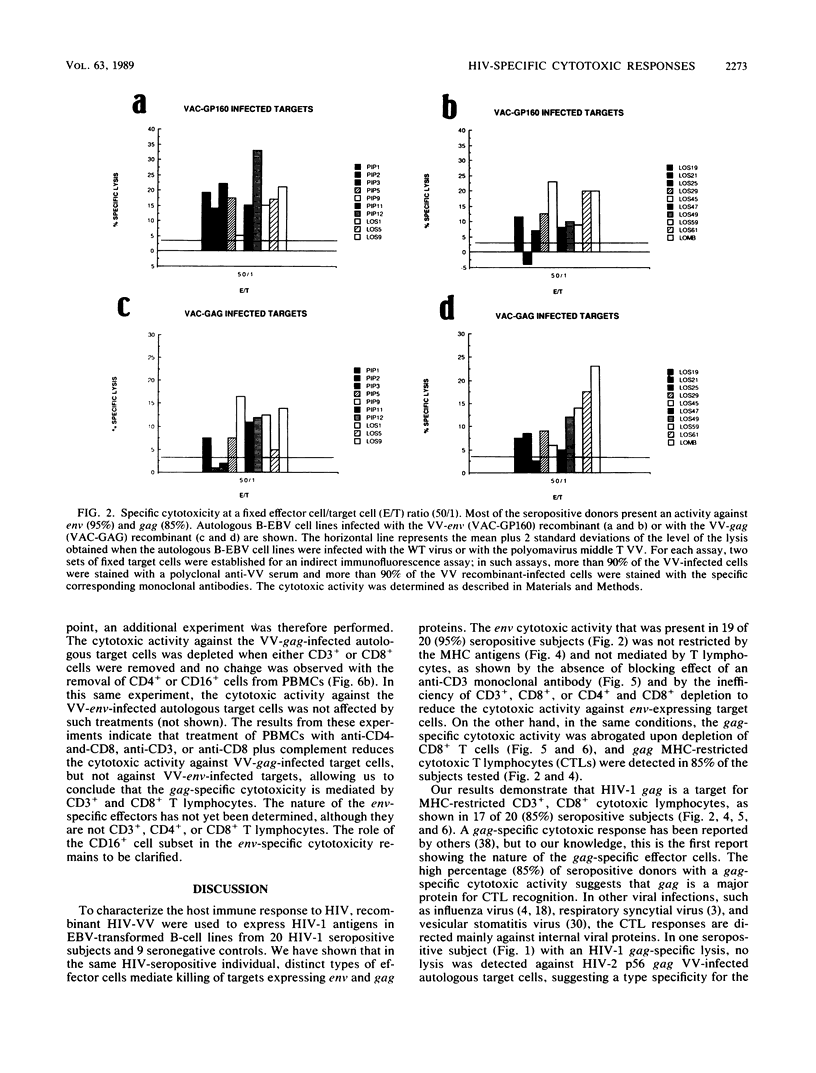
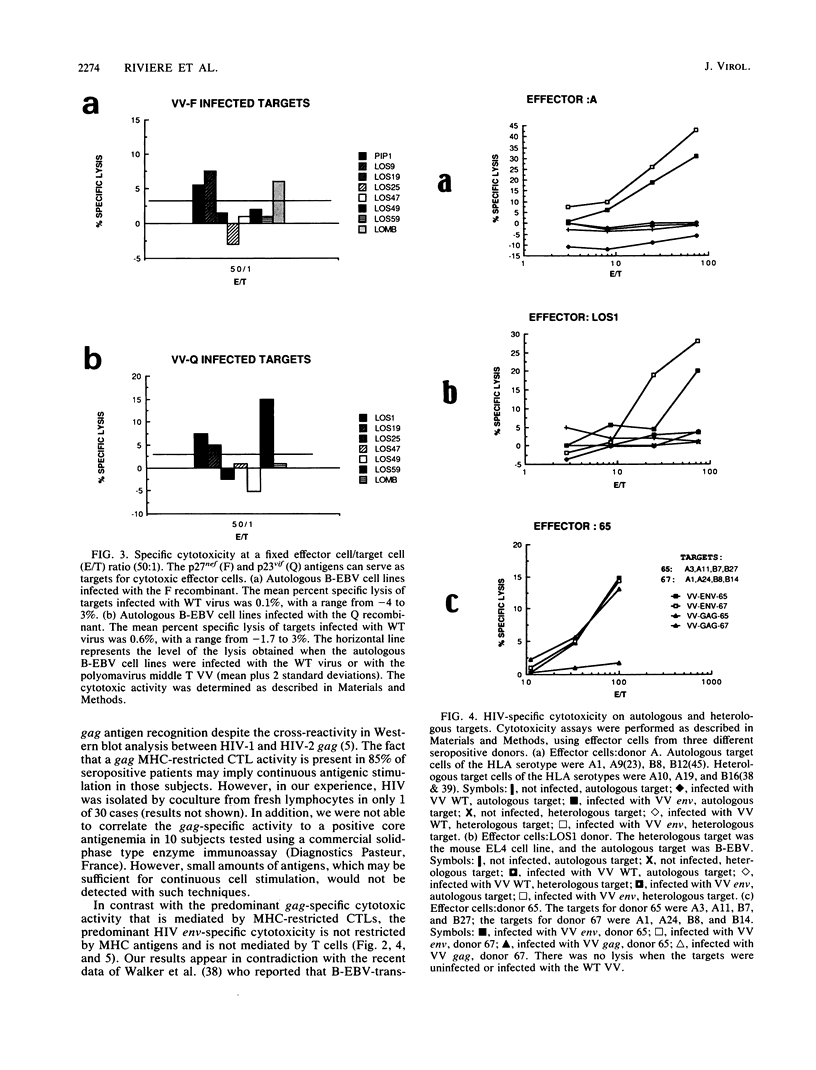
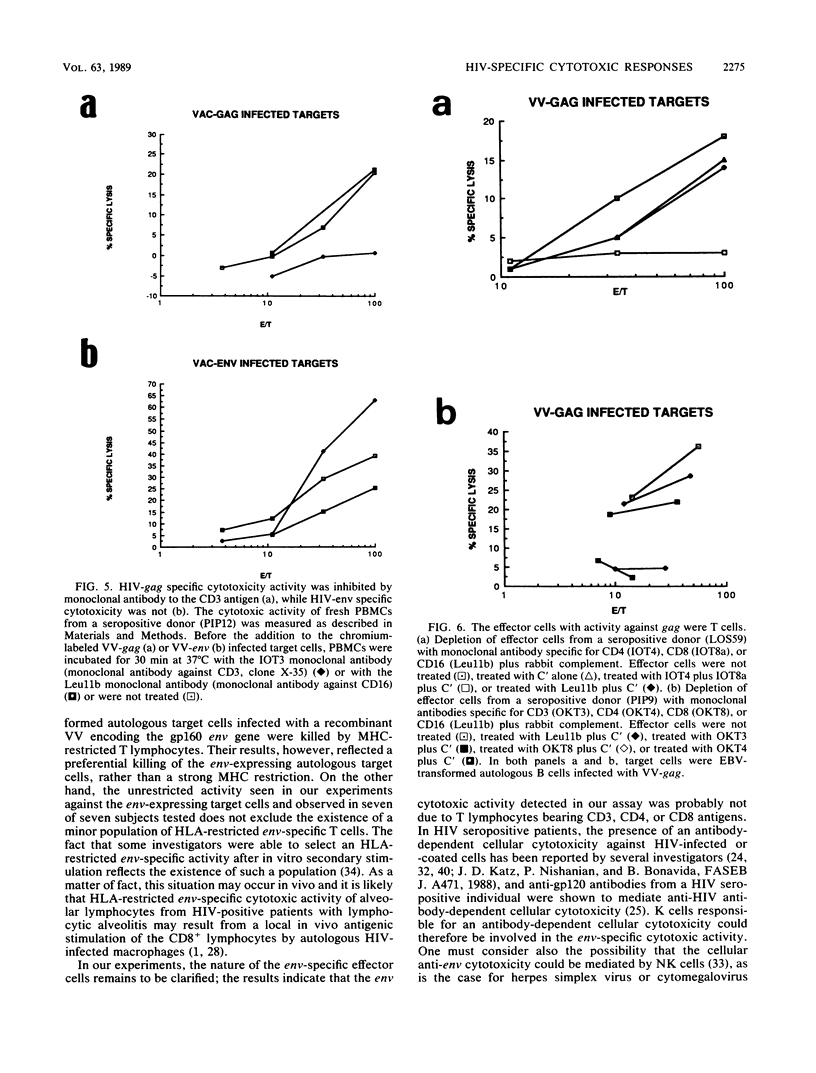
By using target cells that expressed isolated env, gag, p27nef, or p23vif molecules introduced by recombinant vaccinia viruses containing genes encoding these polypeptides, it was possible to identify env, gag, p27nef, and p23vif as cytolytic target antigens for freshly isolated blood cells from human immunodeficiency virus 1 (HIV-1) seropositive patients. Most of the patients tested (95%) manifested a specific cytotoxic activity against vaccinia virus-env-infected target cells. The env-specific cytotoxic activity was not restricted by the major histocompatibility complex and was not mediated by T lymphocytes, as shown by the absence of blocking effect with an anti-CD3 monoclonal antibody and by the inefficiency of CD3+, CD8+, or CD4+ and CD8+ depletion to reduce the cytotoxic activity against the env-expressing target cells. In the same conditions, the cytotoxic activity specific for gag was abrogated and gag major histocompatibility complex-restricted cytotoxic T lymphocytes were detected in 85% of the subjects tested. Therefore, in a HIV-1 seropositive subject, distinct types of effector cells mediate the lysis of target cells expressing gag and env proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autran B., Mayaud C. M., Raphael M., Plata F., Denis M., Bourguin A., Guillon J. M., Debre P., Akoun G. Evidence for a cytotoxic T-lymphocyte alveolitis in human immunodeficiency virus-infected patients. AIDS. 1988 Jun;2(3):179–183. [PubMed] [Google Scholar]
- Bandyopadhyay S., Perussia B., Trinchieri G., Miller D. S., Starr S. E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986 Jul 1;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987 Apr;61(4):1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Gilden D. H., Monjan A. A., Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971 Nov-Dec;30(6):1831–1841. [PubMed] [Google Scholar]
- Cullen B. R., Hauber J., Campbell K., Sodroski J. G., Haseltine W. A., Rosen C. A. Subcellular localization of the human immunodeficiency virus trans-acting art gene product. J Virol. 1988 Jul;62(7):2498–2501. doi: 10.1128/jvi.62.7.2498-2501.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Mitsuyasu R., Gottlieb M. S., Lebow L. T., Bonavida B. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J Immunol. 1987 Jul 1;139(1):55–60. [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Rivière Y., Dott K., Schmitt D., Girard M., Montagnier L., Lecocq J. Improved antigenicity of the HIV env protein by cleavage site removal. Protein Eng. 1988 Sep;2(3):219–225. doi: 10.1093/protein/2.3.219. [DOI] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Gerlinger P., Clertant P., Guizani I., Cuzin F., Chambon P. Tumour prevention and rejection with recombinant vaccinia. 1987 Apr 30-May 6Nature. 326(6116):878–880. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Rüedi E., Zinkernagel R. M. Virus-triggered immune suppression in mice caused by virus-specific cytotoxic T cells. J Exp Med. 1988 May 1;167(5):1749–1754. doi: 10.1084/jem.167.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann R. A., Golub S. H., Park N. H. HSV-1-infected oral epithelial cells are targets for natural killer cells. J Dent Res. 1987 Mar;66(3):770–773. doi: 10.1177/00220345870660031301. [DOI] [PubMed] [Google Scholar]
- Ljunggren K., Böttiger B., Biberfeld G., Karlson A., Fenyö E. M., Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus. Presence at different clinical stages. J Immunol. 1987 Oct 1;139(7):2263–2267. [PubMed] [Google Scholar]
- Lyerly H. K., Reed D. L., Matthews T. J., Langlois A. J., Ahearne P. A., Petteway S. R., Jr, Weinhold K. J. Anti-GP 120 antibodies from HIV seropositive individuals mediate broadly reactive anti-HIV ADCC. AIDS Res Hum Retroviruses. 1987;3(4):409–422. doi: 10.1089/aid.1987.3.409. [DOI] [PubMed] [Google Scholar]
- Montagnier L. Lymphadenopathy associated virus: its role in the pathogenesis of AIDS and related diseases. Prog Allergy. 1986;37:46–64. [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Ruscetti F. W., Mikovits J. A., Kalyanaraman V. S., Overton R., Stevenson H., Stromberg K., Herberman R. B., Farrar W. L., Ortaldo J. R. Analysis of effector mechanisms against HTLV-I- and HTLV-III/LAV-infected lymphoid cells. J Immunol. 1986 May 15;136(10):3619–3624. [PubMed] [Google Scholar]
- Shepp D. H., Daguillard F., Mann D., Quinnan G. V. Human class I MHC-restricted cytotoxic T-lymphocytes specific for human immunodeficiency virus envelope antigens. AIDS. 1988 Apr;2(2):113–117. doi: 10.1097/00002030-198804000-00007. [DOI] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Matthews T. J., Tyler D. S., Ahearne P. M., Stine K. C., Langlois A. J., Durack D. T., Bolognesi D. P. Cellular anti-GP120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988 Apr 23;1(8591):902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- Whitton J. L., Gebhard J. R., Lewicki H., Tishon A., Oldstone M. B. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988 Mar;62(3):687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Southern P. J., Oldstone M. B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988 Feb;162(2):321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]


