Abstract
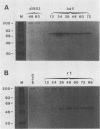
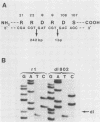
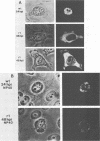
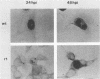
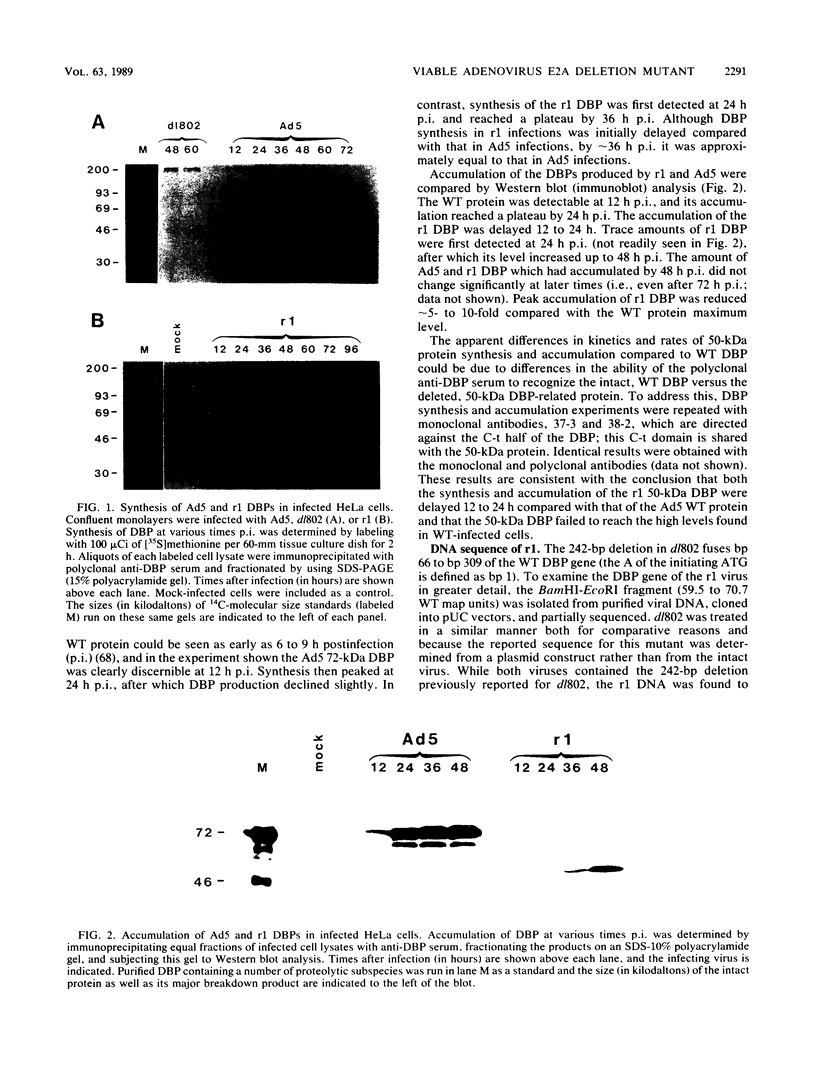
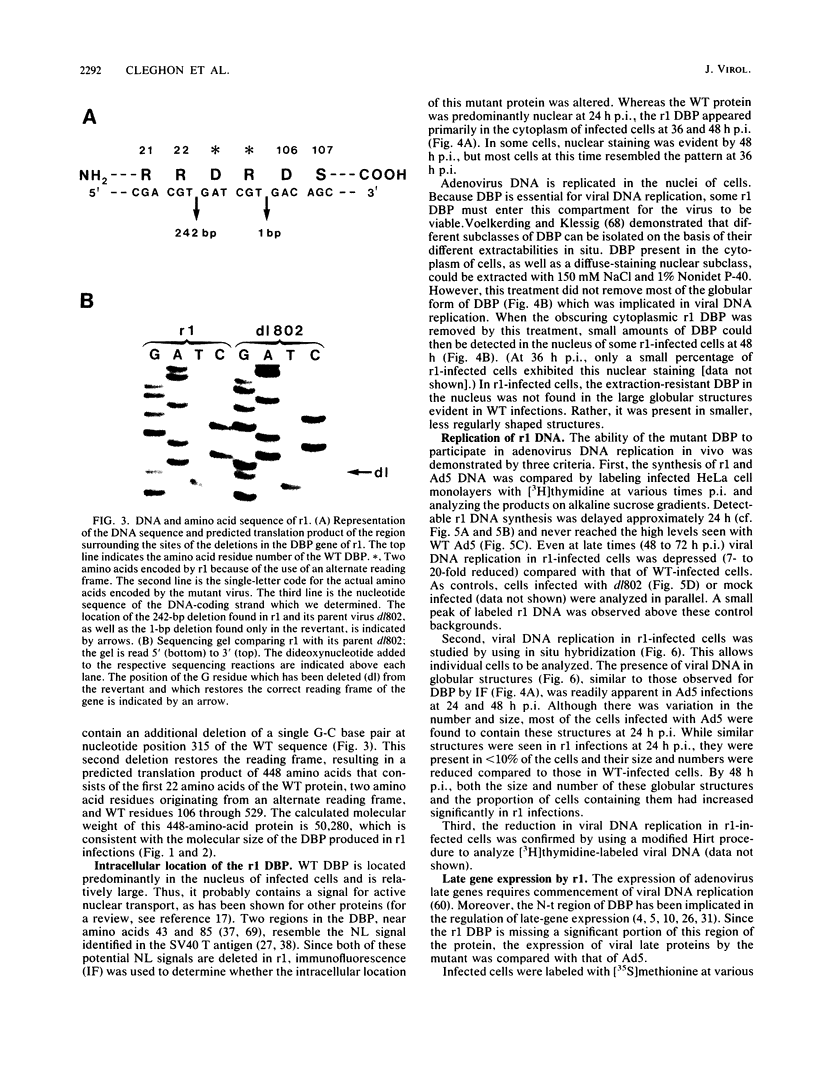
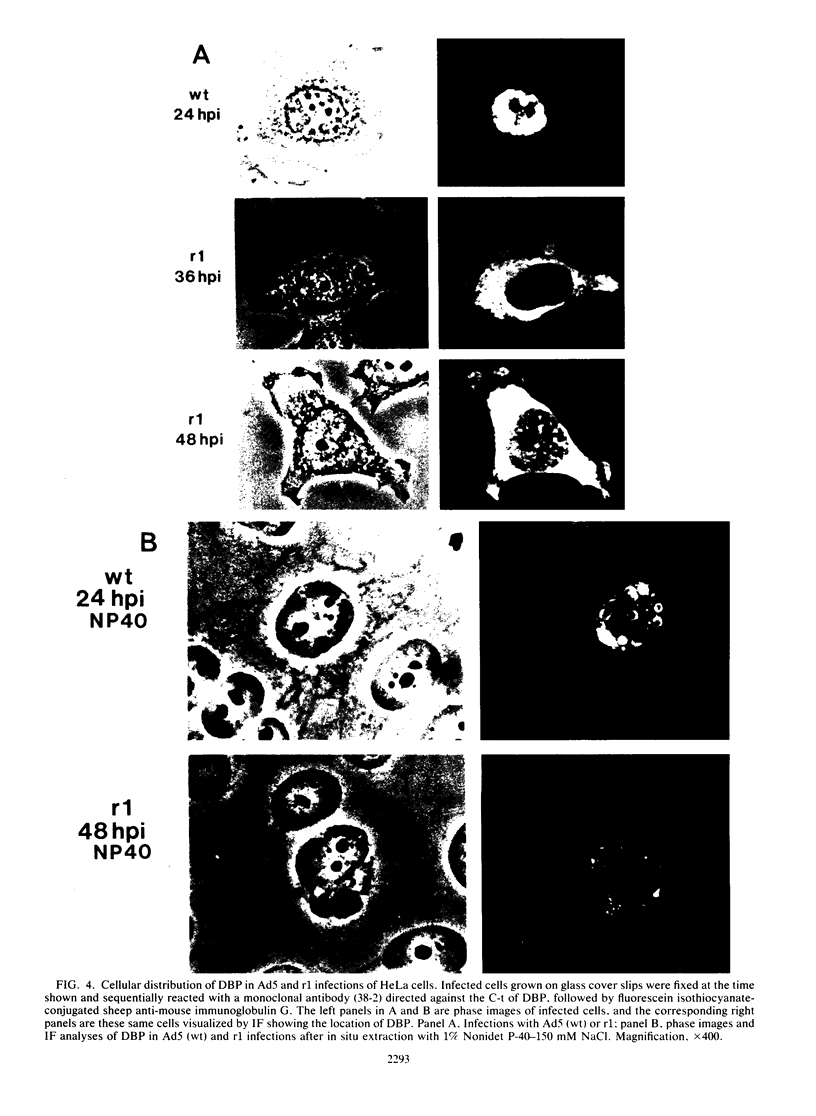
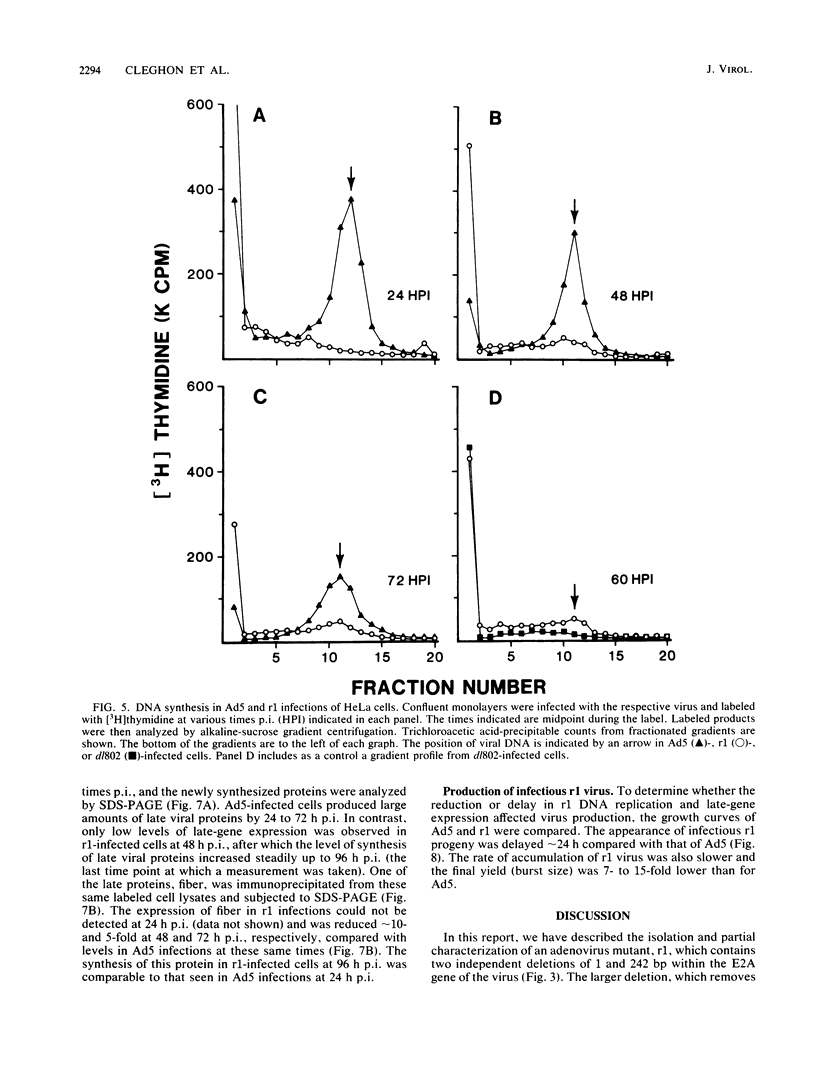
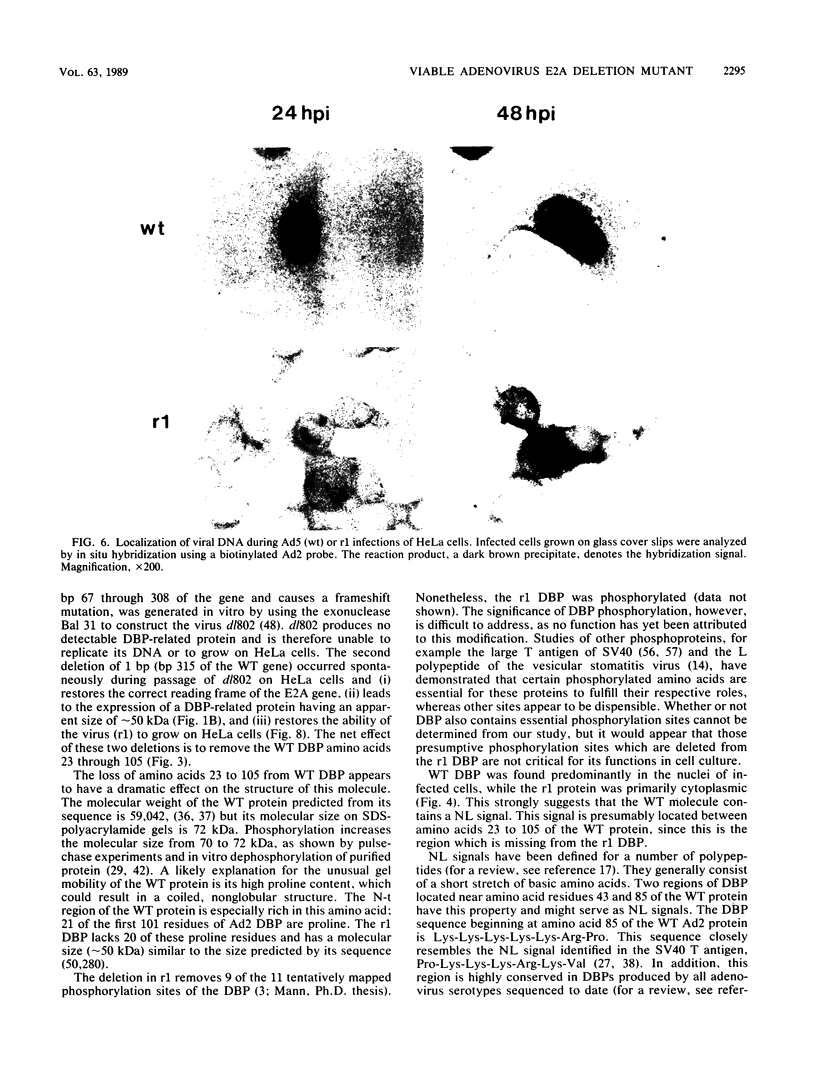
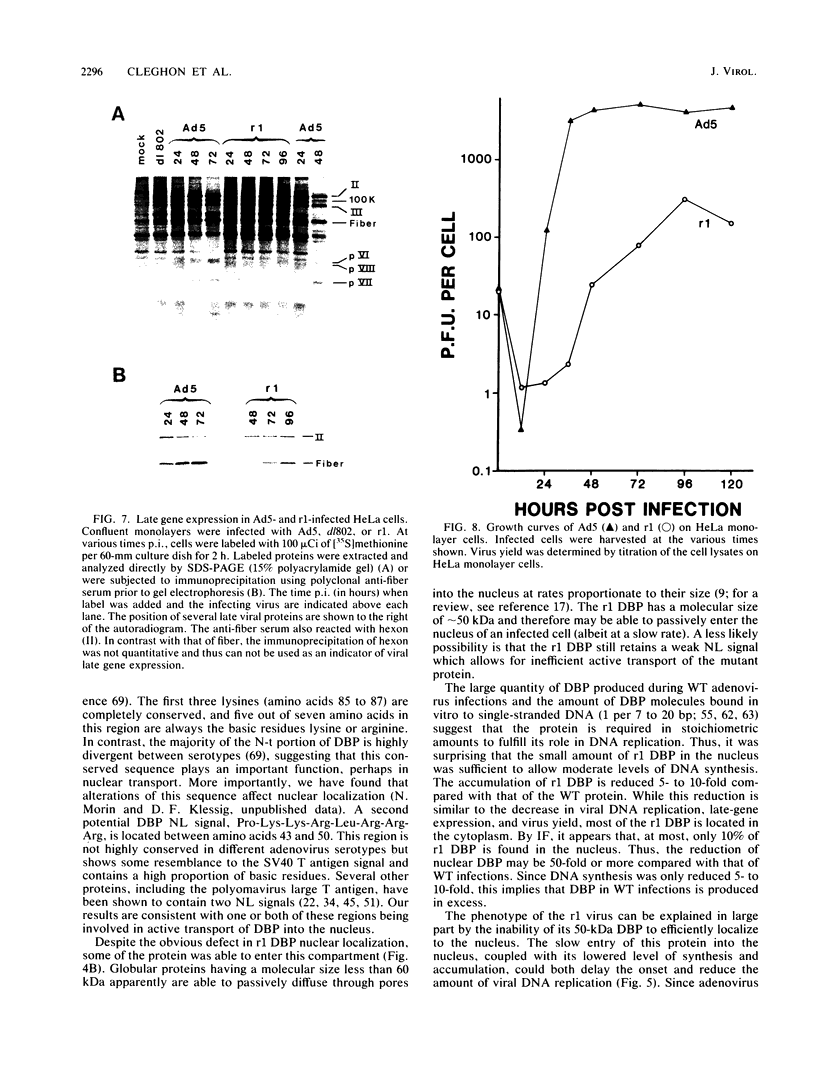
The isolation and characterization of an adenovirus mutant, Ad5dl802r1, containing two independent deletions in the 72-kilodalton (kDa) DNA-binding protein (DBP) gene is described. The two deletions remove amino acids 23 through 105 of DBP, resulting in the production of a 50-kDa product. Expression of this truncated DBP was delayed 12 to 24 h compared with that of the 72-kDa protein produced by wild-type adenovirus type 5. The DBP was located primarily in the cytoplasm of infected cells, whereas the wild-type product was predominantly nuclear. Therefore, DBP appears to contain a nuclear localization signal within the deleted region. Ad5dl802r1 DNA synthesis, viral late gene expression, and virus production were all delayed 12 to 24 h and were approximately 10-fold lower than with wild-type adenovirus type 5. These phenotypic properties can be accounted for by the delay in synthesis and the inefficient accumulation of the 50-kDa DBP within the nucleus of infected cells. The truncated DBP also lacks the majority of amino acids which are phosphorylated in the normal protein. The loss of these phosphorylation sites does not appear to seriously impair the ability of the protein to carry out its functions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1987 Oct;61(10):3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Hardy M. M., Dunn J. J., Klessig D. F. Independent, spontaneous mutants of adenovirus type 2-simian virus 40 hybrid Ad2+ND3 that grow efficiently in monkey cells possess indentical mutations in the adenovirus type 2 DNA-binding protein gene. J Virol. 1983 Oct;48(1):31–39. doi: 10.1128/jvi.48.1.31-39.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Ariga H., Klein H., Levine A. J., Horwitz M. S. A cleavage product of the adenovirus DNA binding protein is active in DNA replication in vitro. Virology. 1980 Feb;101(1):307–310. doi: 10.1016/0042-6822(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Babich A., Nevins J. R. The stability of early adenovirus mRNA is controlled by the viral 72 kd DNA-binding protein. Cell. 1981 Nov;26(3 Pt 1):371–379. doi: 10.1016/0092-8674(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Autoregulation of adenovirus type 5 early gene expression II. Effect of temperature-sensitive early mutations on virus RNA accumulation. J Virol. 1978 Nov;28(2):450–456. doi: 10.1128/jvi.28.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Cleghon V. G., Klessig D. F. Association of the adenovirus DNA-binding protein with RNA both in vitro and in vivo. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8947–8951. doi: 10.1073/pnas.83.23.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walser A., Klockmann U. A subclass of the adenovirus 72K DNA binding protein specifically associating with the cytoskeletal framework of the plasma membrane. Virology. 1988 Aug;165(2):457–468. doi: 10.1016/0042-6822(88)90589-2. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Lord S. T., Linné T., Pettersson U., Philipson L. Interaction between the adenovirus DNA-binding protein and double-stranded DNA. J Mol Biol. 1979 Aug 5;132(2):163–180. doi: 10.1016/0022-2836(79)90389-9. [DOI] [PubMed] [Google Scholar]
- Friefeld B. R., Krevolin M. D., Horwitz M. S. Effects of the adenovirus H5ts125 and H5ts107 DNA binding proteins on DNA replication in vitro. Virology. 1983 Jan 30;124(2):380–389. doi: 10.1016/0042-6822(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Greenspan D., Palese P., Krystal M. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J Virol. 1988 Aug;62(8):3020–3026. doi: 10.1128/jvi.62.8.3020-3026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kingston R. E., Sharp P. A. Inhibition of adenovirus early region IV transcription in vitro by a purified viral DNA binding protein. Nature. 1983 Apr 7;302(5908):545–547. doi: 10.1038/302545a0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Anderson K. P., Klessig D. F. Partial block to transcription of human adenovirus type 2 late genes in abortively infected monkey cells. J Virol. 1985 Nov;56(2):378–385. doi: 10.1128/jvi.56.2.378-385.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R. Sequence of the DNA-binding protein of a human subgroup E adenovirus (type 4): comparisons with subgroup A (type 12), subgroup B (type 7), and subgroup C (type 5). Virology. 1985 Oct 15;146(1):90–101. doi: 10.1016/0042-6822(85)90055-8. [DOI] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T., Cleghon V. Construction of human cell lines which contain and express the adenovirus DNA binding protein gene by cotransformation with the HSV-1 tk gene. Virus Res. 1984;1(2):169–188. doi: 10.1016/0168-1702(84)90071-6. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Quinlan M. P. Genetic evidence for separate functional domains on the human adenovirus specified, 72 kd, DNA binding protein. J Mol Appl Genet. 1982;1(4):263–272. [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Jones N., Rosenberg M., Westphal H. Mapping of functional domains in adenovirus E1A proteins. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7480–7484. doi: 10.1073/pnas.82.22.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Nicolas J. C., van Schaik F. M., Sussenbach J. S. Structure and function of DNA binding proteins from revertants of adenovirus type 5 mutants with a temperature-sensitive DNA replication. Virology. 1983 Jan 30;124(2):425–433. doi: 10.1016/0042-6822(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Van Schaik F. M., Sussenbach J. S. Nucleotide sequence of the gene encoding adenovirus type 2 DNA binding protein. Nucleic Acids Res. 1982 Aug 11;10(15):4493–4500. doi: 10.1093/nar/10.15.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Structure and organization of the gene coding for the DNA binding protein of adenovirus type 5. Nucleic Acids Res. 1981 Sep 25;9(18):4439–4457. doi: 10.1093/nar/9.18.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Levinson A., Levine A. J. The isolation and identification of the adenovirus group C tumor antigens. Virology. 1977 Jan;76(1):1–11. doi: 10.1016/0042-6822(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Linné T., Jörnvall H., Philipson L. Purification and characterization of the phosphorylated DNA-binding protein from adenovirus-type-2-infected cells. Eur J Biochem. 1977 Jun 15;76(2):481–490. doi: 10.1111/j.1432-1033.1977.tb11618.x. [DOI] [PubMed] [Google Scholar]
- Linné T., Philipson L. Further characterization of the phosphate moiety of the adenovirus type 2 DNA-binding protein. Eur J Biochem. 1980 Jan;103(2):259–270. doi: 10.1111/j.1432-1033.1980.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Sarnow P., Girard M., Levine A. J. Host range temperature-conditional mutants in the adenovirus DNA binding protein are defective in the assembly of infectious virus. Virology. 1983 Apr 15;126(1):228–239. doi: 10.1016/0042-6822(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C. O., Kitchingman G. R. Sequence of the DNA-binding protein gene of a human subgroup B adenovirus (type 7). Comparisons with subgroup C (type 5) and subgroup A (type 12). J Biol Chem. 1984 Apr 25;259(8):5003–5009. [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985 Dec;56(3):767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. The function(s) provided by the adenovirus-specified, DNA-binding protein required for viral late gene expression is independent of the role of the protein in viral DNA replication. J Virol. 1984 Jan;49(1):35–49. doi: 10.1128/jvi.49.1.35-49.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F., Williams J. Multiple effects of the 72-kDa, adenovirus-specified DNA binding protein on the efficiency of cellular transformation. Virology. 1987 Feb;156(2):366–376. doi: 10.1016/0042-6822(87)90416-8. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Carter B. J., Westphal H. Vero cells injected with adenovirus type 2 mRNA produce authentic viral polypeptide patterns: early mRNA promotes growth of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1980 Feb;77(2):931–935. doi: 10.1073/pnas.77.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981 Nov;27(1 Pt 2):133–141. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- Rossini M. The role of adenovirus early region 1A in the regulation of early regions 2A and 1B expression. Virology. 1983 Nov;131(1):49–58. doi: 10.1016/0042-6822(83)90532-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J Virol. 1988 May;62(5):1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Wold W. S., Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J Virol. 1977 May;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Tsernoglou D., Tsugita A., Tucker A. D., van der Vliet P. C. Characterization of the chymotryptic core of the adenovirus DNA-binding protein. FEBS Lett. 1985 Sep 2;188(2):248–252. doi: 10.1016/0014-5793(85)80381-1. [DOI] [PubMed] [Google Scholar]
- Van der Vliet P. C., Zandberg J., Jansz H. S. Evidence for a function of the adenovirus DNA-binding protein in initiation in DNA synthesis as well as in elongation of nascent DNA chains. Virology. 1977 Jul 1;80(1):98–110. doi: 10.1016/0042-6822(77)90383-x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos H. L., van der Lee F. M., Reemst A. M., van Loon A. E., Sussenbach J. S. The genes encoding the DNA binding protein and the 23K protease of adenovirus types 40 and 41. Virology. 1988 Mar;163(1):1–10. doi: 10.1016/0042-6822(88)90227-9. [DOI] [PubMed] [Google Scholar]
- van Amerongen H., van Grondelle R., van der Vliet P. C. Interaction between adenovirus DNA-binding protein and single-stranded polynucleotides studied by circular dichroism and ultraviolet absorption. Biochemistry. 1987 Jul 28;26(15):4646–4652. doi: 10.1021/bi00389a009. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]