Abstract
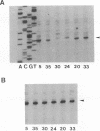
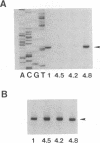
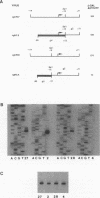
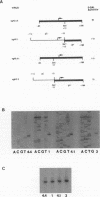
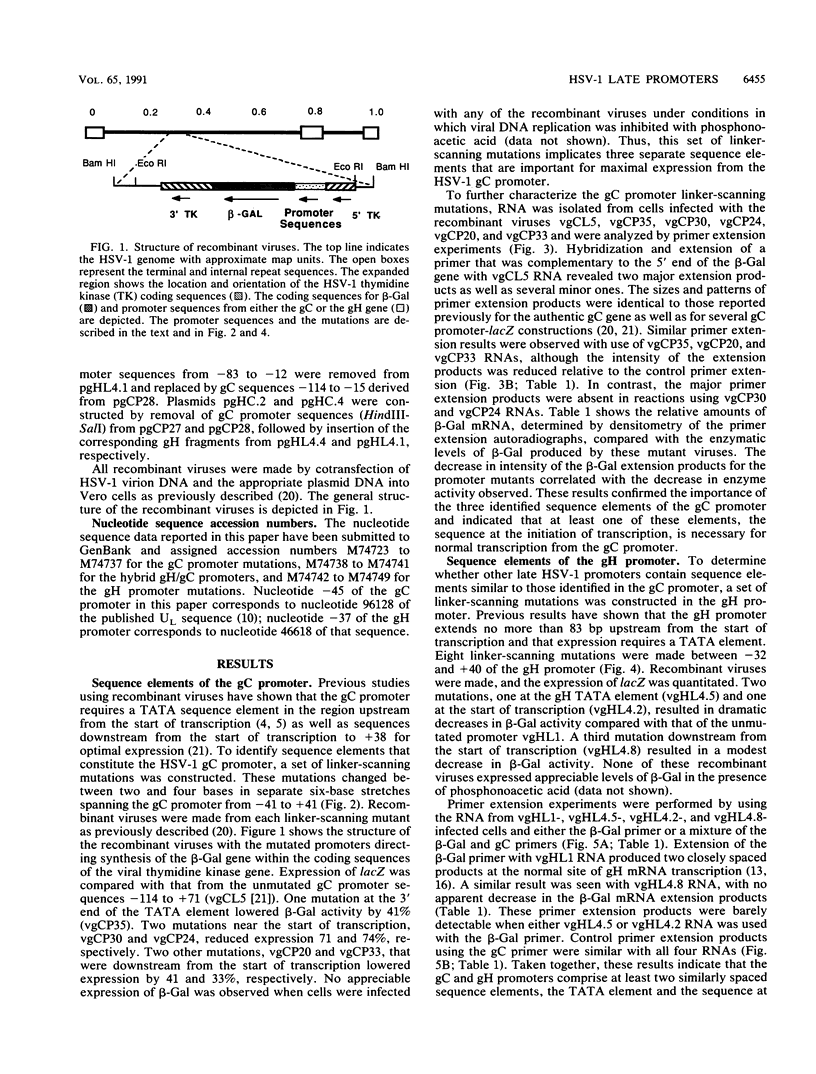
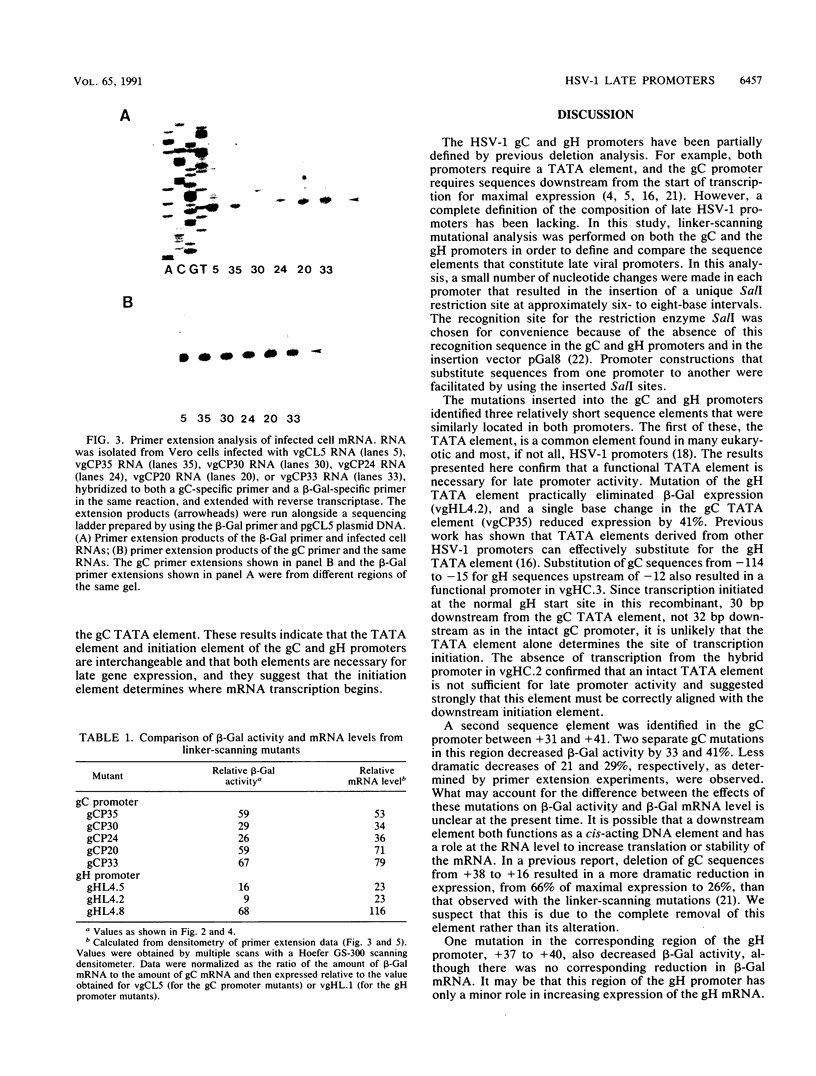
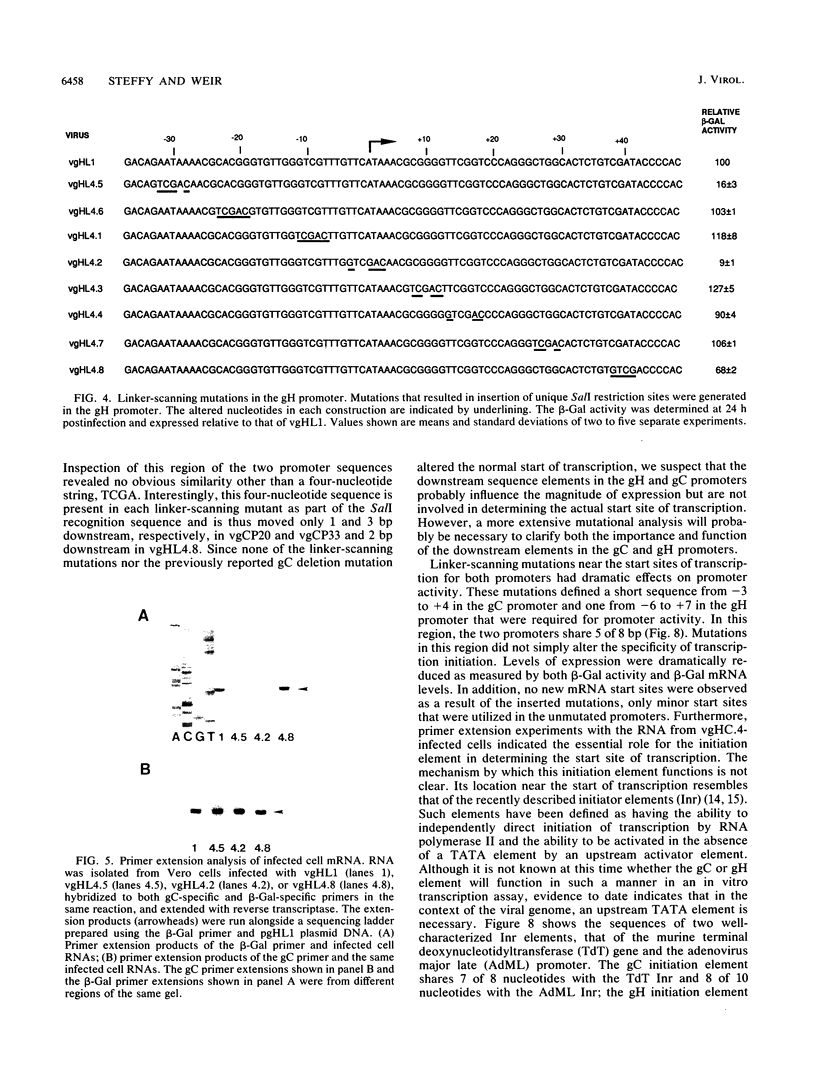
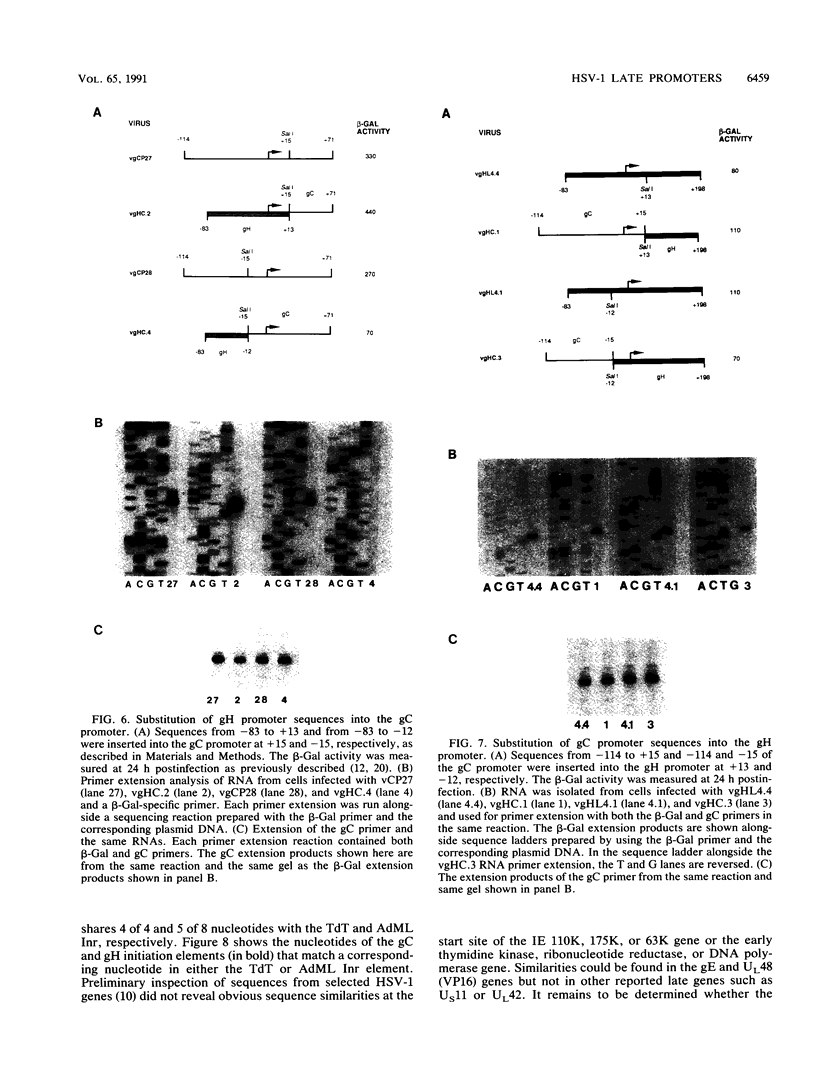
To investigate the cis-acting sequence elements that are involved in the regulation of herpes simplex virus type 1 late gene expression, linker-scanning mutations were constructed in the promoters of the glycoprotein C and glycoprotein H genes. Each promoter mutation was inserted upstream of the Escherichia coli lacZ gene in a recombinant virus, and the relative activities of beta-galactosidase expressed from individual recombinant viruses were compared. This analysis identified three sequence elements in each promoter: a TATA element, an element that overlapped the start of transcription, and an element downstream from the start of transcription. Primer extension analysis confirmed these results and showed that mutations in either the TATA element or the initiation sequence could eliminate normal transcription initiation. Analysis of expression from hybrid promoters revealed that the TATA and the initiation elements were interchangeable, at least when correctly aligned, and that the initiation element plays a pivotal role in determining the actual site of transcription initiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole C. N., Stacy T. P. Identification of sequences in the herpes simplex virus thymidine kinase gene required for efficient processing and polyadenylation. Mol Cell Biol. 1985 Aug;5(8):2104–2113. doi: 10.1128/mcb.5.8.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mavromara-Nazos P., Roizman B. Delineation of regulatory domains of early (beta) and late (gamma 2) genes by construction of chimeric genes expressed in herpes simplex virus 1 genomes. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4071–4075. doi: 10.1073/pnas.86.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Michael N., Spector D., Mavromara-Nazos P., Kristie T. M., Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988 Mar 25;239(4847):1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- Sharp J. A., Wagner M. J., Summers W. C. Transcription of herpes simplex virus genes in vivo: overlap of a late promoter with the 3' end of the early thymidine kinase gene. J Virol. 1983 Jan;45(1):10–17. doi: 10.1128/jvi.45.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffy K. R., Weir J. P. Upstream promoter elements of the herpes simplex virus type 1 glycoprotein H gene. J Virol. 1991 Feb;65(2):972–975. doi: 10.1128/jvi.65.2.972-975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Narayanan P. R. Expression of the herpes simplex virus type 1 glycoprotein C gene requires sequences in the 5' noncoding region of the gene. J Virol. 1990 Jan;64(1):445–449. doi: 10.1128/jvi.64.1.445-449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Narayanan P. R. The use of beta-galactosidase as a marker gene to define the regulatory sequences of the herpes simplex virus type 1 glycoprotein C gene in recombinant herpesviruses. Nucleic Acids Res. 1988 Nov 11;16(21):10267–10282. doi: 10.1093/nar/16.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Steffy K. R., Sethna M. An insertion vector for the analysis of gene expression during herpes simplex virus infection. Gene. 1990 May 14;89(2):271–274. doi: 10.1016/0378-1119(90)90016-k. [DOI] [PubMed] [Google Scholar]
- Zhang F., Denome R. M., Cole C. N. Fine-structure analysis of the processing and polyadenylation region of the herpes simplex virus type 1 thymidine kinase gene by using linker scanning, internal deletion, and insertion mutations. Mol Cell Biol. 1986 Dec;6(12):4611–4623. doi: 10.1128/mcb.6.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]