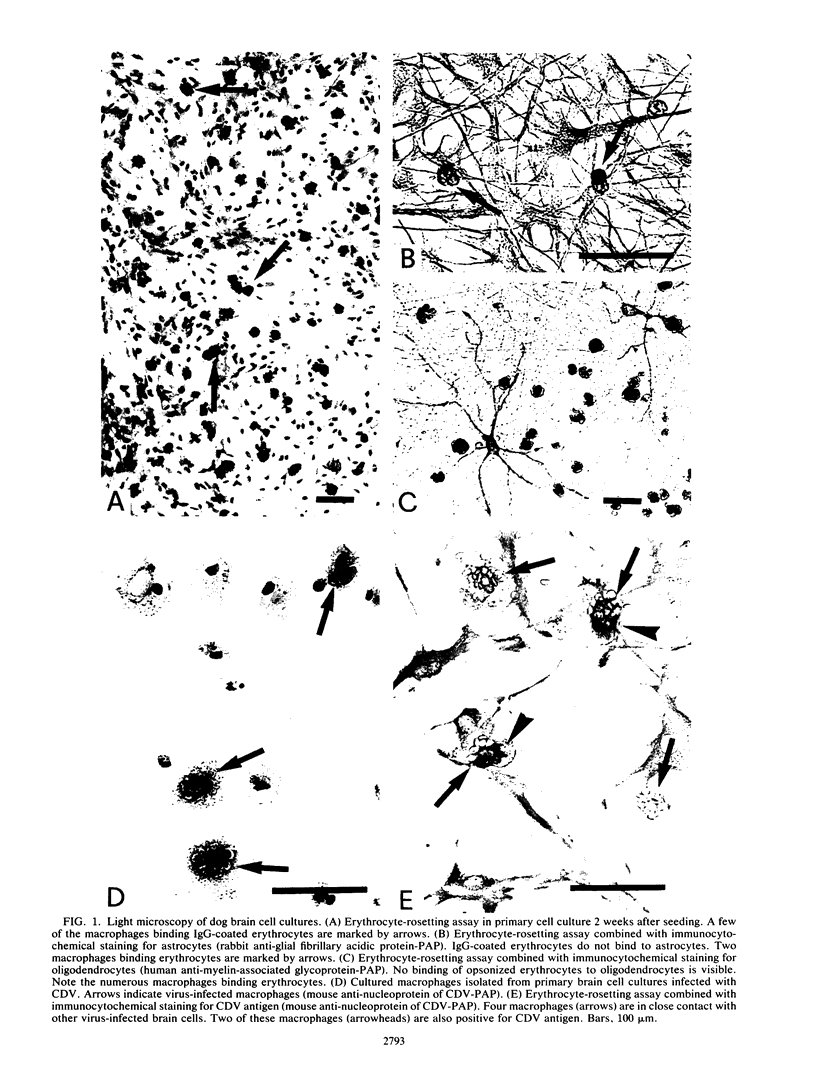
Abstract
Canine distemper is characterized mainly by respiratory, enteric, and nervous symptoms. Infection of the central nervous system results in demyelination, to which inflammation has been shown to contribute significantly. It has been proposed that macrophages play a major role as effector cells in this process. We report that cultured dog brain cells contain a population of macrophages capable of producing reactive oxygen species as measured by luminol-dependent chemiluminescence. In cultures infected with canine distemper virus, a burst of reactive oxygen is triggered by antiviral antibody. This response depends on the presence of viral antigens on the surfaces of infected cells and is mediated by the interaction of antigen-bound antibody with Fc receptors on the macrophages. Since there is no evidence in vitro or in vivo that oligodendrocytes, the cells forming myelin, are infected, our observation supports the hypothesis that "innocent bystander killing" is important in demyelination caused by canine distemper virus. Reactive oxygen species released from macrophages may contribute to destruction of myelin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
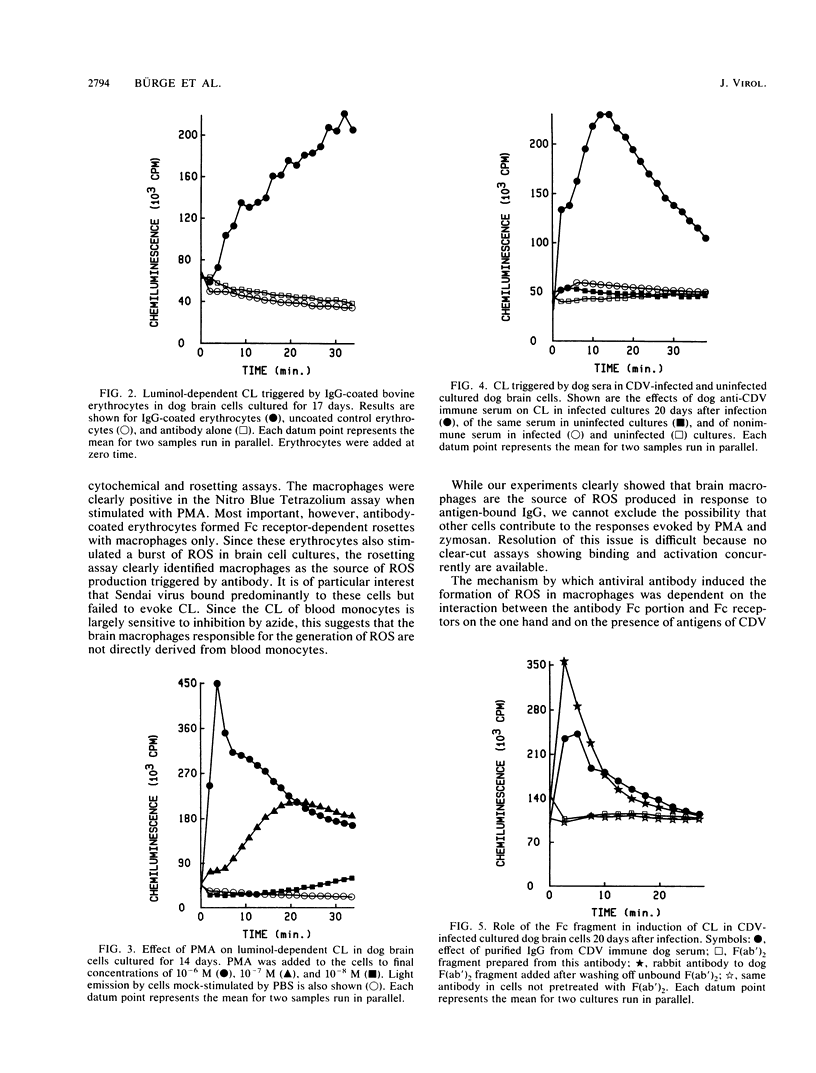
- Allen R. C. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- Bingham E. L., Fenger T. W., Sugar A., Smith J. W. Dependence on antibody for induction of chemiluminescence in polymorphonuclear leukocytes by herpes simplex virus. Invest Ophthalmol Vis Sci. 1985 Sep;26(9):1236–1243. [PubMed] [Google Scholar]
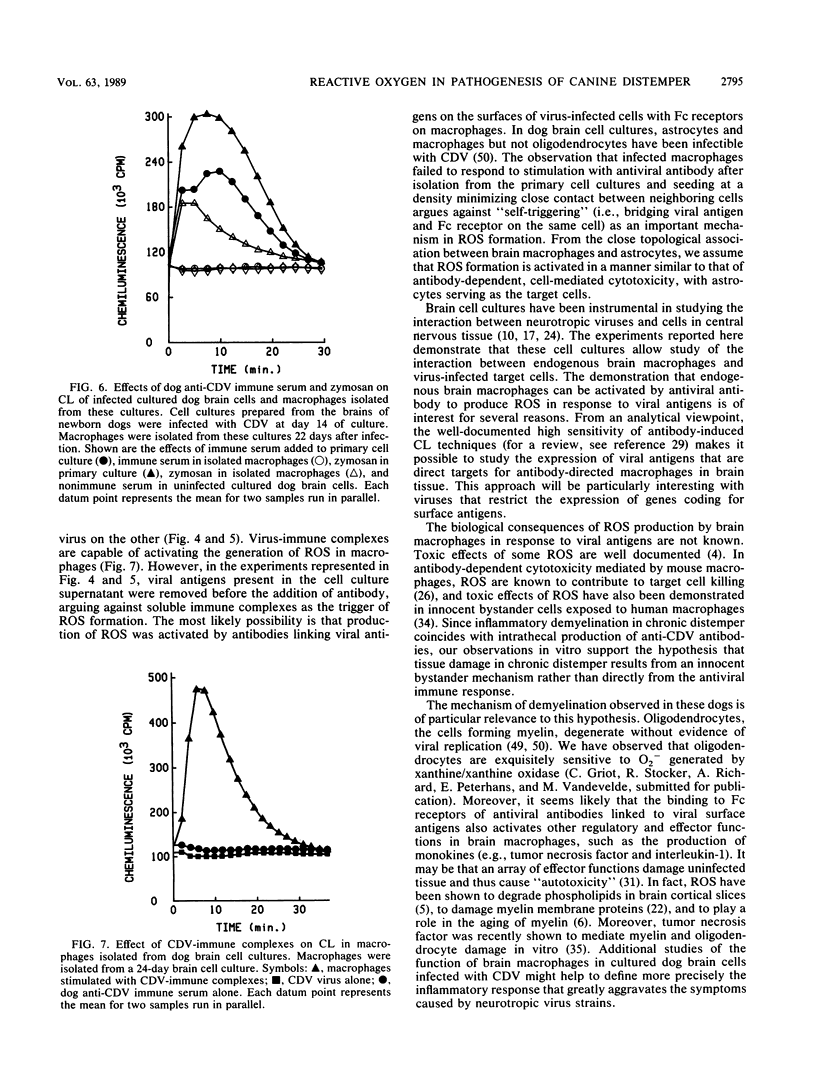
- Bollo E., Zurbriggen A., Vandevelde M., Fankhauser R. Canine distemper virus clearance in chronic inflammatory demyelination. Acta Neuropathol. 1986;72(1):69–73. doi: 10.1007/BF00687949. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Yurko M., Fishman R. A. Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J Neurochem. 1982 Feb;38(2):525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x. [DOI] [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Moscarello M. A. Disorder in human myelin induced by superoxide radical: an in vitro investigation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):141–146. doi: 10.1016/0006-291x(83)91552-8. [DOI] [PubMed] [Google Scholar]
- Colton C. A., Gilbert D. L. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987 Nov 2;223(2):284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- Ditrich H. Brain macrophages in cerebellar cell cultures. Tissue Cell. 1986;18(5):645–658. doi: 10.1016/0040-8166(86)90067-4. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey H., Pfister H., Messerli J., Sturzenegger N., Grolimund F. Methods of isolation, purification and quantitation of bovine immunoglobulins: a technical review. Zentralbl Veterinarmed B. 1976 May;23(4):269–300. doi: 10.1111/j.1439-0450.1976.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986 Dec 1;137(11):3521–3527. [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Baker T. J., Shih L. C., Lachman L. B. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986 Aug 1;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Bologa L., Siegel L., Londe H. Theiler's virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15(4):491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. The polypeptides of canine distemper virus: synthesis in infected cells and relatedness to the polypeptides of other morbilliviruses. Virology. 1980 Jan 30;100(2):433–449. doi: 10.1016/0042-6822(80)90534-6. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Identification of microglia in primary cultures of mixed cerebral cortical cells. Brain Res Bull. 1987 Jul;19(1):153–159. doi: 10.1016/0361-9230(87)90180-8. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Peterhans E. Change in the chemiluminescence reactivity pattern during in vitro differentiation of human monocytes to macrophages. Blut. 1988 May;56(5):213–220. doi: 10.1007/BF00320108. [DOI] [PubMed] [Google Scholar]
- Konat G. W., Wiggins R. C. Effect of reactive oxygen species on myelin membrane proteins. J Neurochem. 1985 Oct;45(4):1113–1118. doi: 10.1111/j.1471-4159.1985.tb05530.x. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Wege H., ter Meulen V. Growth pattern of various JHM coronavirus isolates in primary rat glial cell cultures correlates with differing neurotropism in vivo. Virus Res. 1988 Feb;9(2-3):133–144. doi: 10.1016/0168-1702(88)90028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. L., Debets-Ossenkopp Y., Verbrugh H. A., Verhoef J. Initiation of the respiratory burst of human neutrophils by influenza virus. Infect Immun. 1981 Jun;32(3):1200–1205. doi: 10.1128/iai.32.3.1200-1205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cohn Z. Role of oxygen-dependent mechanisms in antibody-induced lysis of tumor cells by activated macrophages. J Exp Med. 1980 Jul 1;152(1):198–208. doi: 10.1084/jem.152.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhans E., Baechi T., Yewdell J. Evidence for different receptor sites in mouse spleen cells for the Sendai virus hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins. Virology. 1983 Jul 30;128(2):366–376. doi: 10.1016/0042-6822(83)90263-5. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Chemiluminescence: an early event in the interaction of Sendai and influenza viruses with mouse spleen cells. I. The role of the envelope glycoproteins in the stimulation of chemiluminescence. Virology. 1980 Sep;105(2):445–455. doi: 10.1016/0042-6822(80)90045-8. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Sendai virus stimulates chemiluminescence in mouse spleen cells. Biochem Biophys Res Commun. 1979 Nov 14;91(1):383–392. doi: 10.1016/0006-291x(79)90630-2. [DOI] [PubMed] [Google Scholar]
- Raedler E., Raedler A. Local proliferation of brain macrophages in central nervous system tissue cultures. J Neuropathol Exp Neurol. 1984 Sep;43(5):531–540. doi: 10.1097/00005072-198409000-00008. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Rinehart J. J. Human monocyte to macrophage differentiation in vitro: characterization and mechanisms of the increased antibody-dependent cytotoxicity associated with differentiation. J Leukoc Biol. 1984 Feb;35(2):217–228. doi: 10.1002/jlb.35.2.217. [DOI] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sonderer B., Wild P., Wyler R., Fontana A., Peterhans E., Schwyzer M. Murine glia cells in culture can be stimulated to generate reactive oxygen. J Leukoc Biol. 1987 Nov;42(5):463–473. doi: 10.1002/jlb.42.5.463. [DOI] [PubMed] [Google Scholar]
- Summers B. A., Greisen H. A., Appel M. J. Early events in canine distemper demyelinating encephalomyelitis. Acta Neuropathol. 1979 Apr 12;46(1-2):1–10. doi: 10.1007/BF00684797. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987 Jul-Aug;15(3):263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevelde M., Higgins R. J., Kristensen B., Kristensen F., Steck A. J., Kihm U. Demyelination in experimental canine distemper virus infection: immunological, pathologic, and immunohistological studies. Acta Neuropathol. 1982;56(4):285–293. doi: 10.1007/BF00691260. [DOI] [PubMed] [Google Scholar]
- Vandevelde M., Zurbriggen A., Higgins R. J., Palmer D. Spread and distribution of viral antigen in nervous canine distemper. Acta Neuropathol. 1985;67(3-4):211–218. doi: 10.1007/BF00687803. [DOI] [PubMed] [Google Scholar]
- Vandevelde M., Zurbriggen A., Steck A., Bichsel P. Studies on the intrathecal humoral immune response in canine distemper encephalitis. J Neuroimmunol. 1986 Mar;11(1):41–51. doi: 10.1016/0165-5728(86)90073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L., Peterhans E. Stimulation of chemiluminescence in bovine polymorphonuclear leucocytes by virus-antibody complexes and by antibody-coated infected cells. Immunobiology. 1983 May;164(5):333–342. doi: 10.1016/S0171-2985(83)80029-1. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H., Raine C. S., Kay W. J. Observations on viral demyelinating encephalomyelitis. Canine distemper. Lab Invest. 1972 May;26(5):589–599. [PubMed] [Google Scholar]
- Zucker-Franklin D., Warfel A., Grusky G., Frangione B., Teitel D. Novel monocyte-like properties of microglial/astroglial cells. Constitutive secretion of lysozyme and cystatin-C. Lab Invest. 1987 Aug;57(2):176–185. [PubMed] [Google Scholar]
- Zurbriggen A., Vandevelde M., Beranek C. F., Steck A. Morphological and immunocytochemical characterisation of mixed glial cell cultures derived from neonatal canine brain. Res Vet Sci. 1984 May;36(3):270–275. [PubMed] [Google Scholar]
- Zurbriggen A., Vandevelde M., Bollo E. Demyelinating, non-demyelinating and attenuated canine distemper virus strains induce oligodendroglial cytolysis in vitro. J Neurol Sci. 1987 Jun;79(1-2):33–41. doi: 10.1016/0022-510x(87)90257-7. [DOI] [PubMed] [Google Scholar]
- Zurbriggen A., Vandevelde M., Dumas M. Secondary degeneration of oligodendrocytes in canine distemper virus infection in vitro. Lab Invest. 1986 Apr;54(4):424–431. [PubMed] [Google Scholar]