Abstract
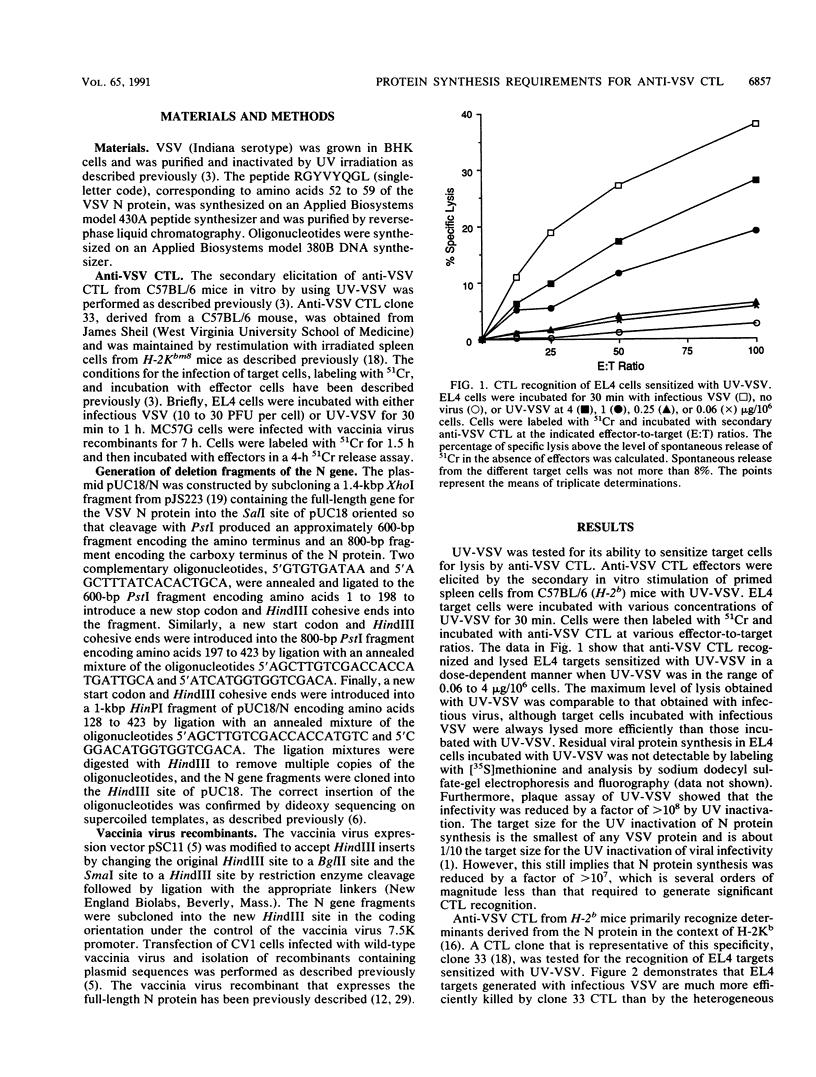
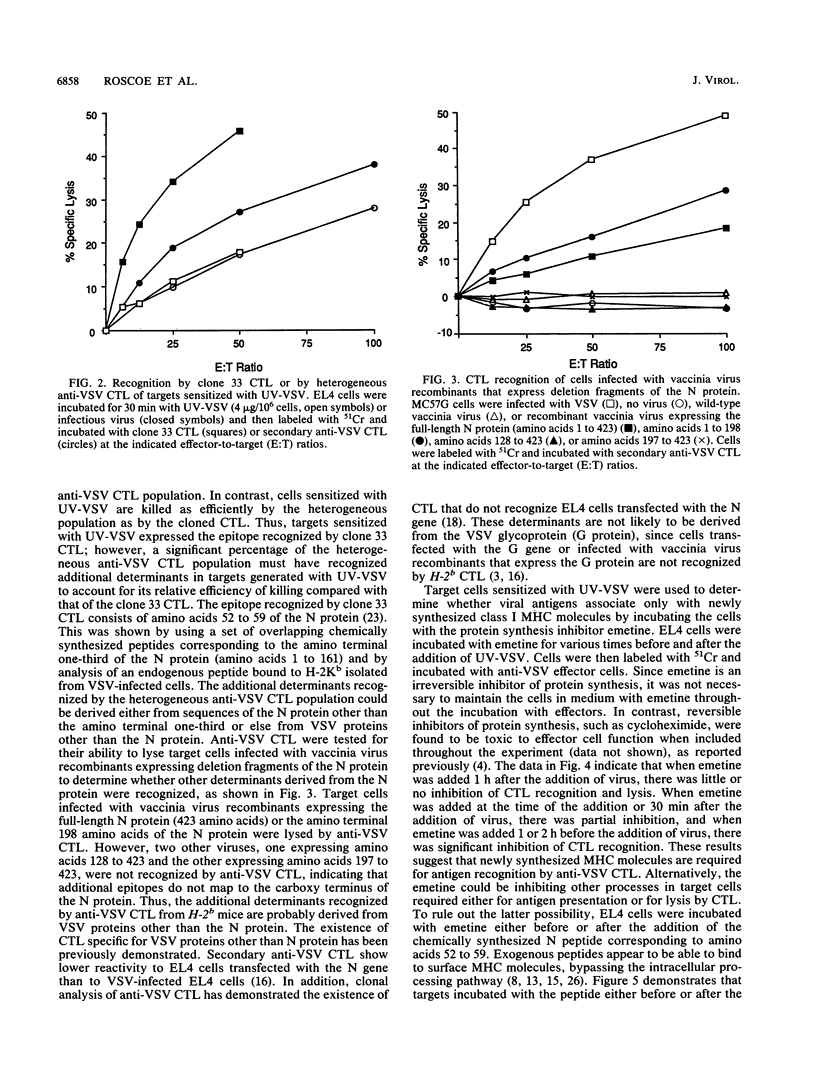
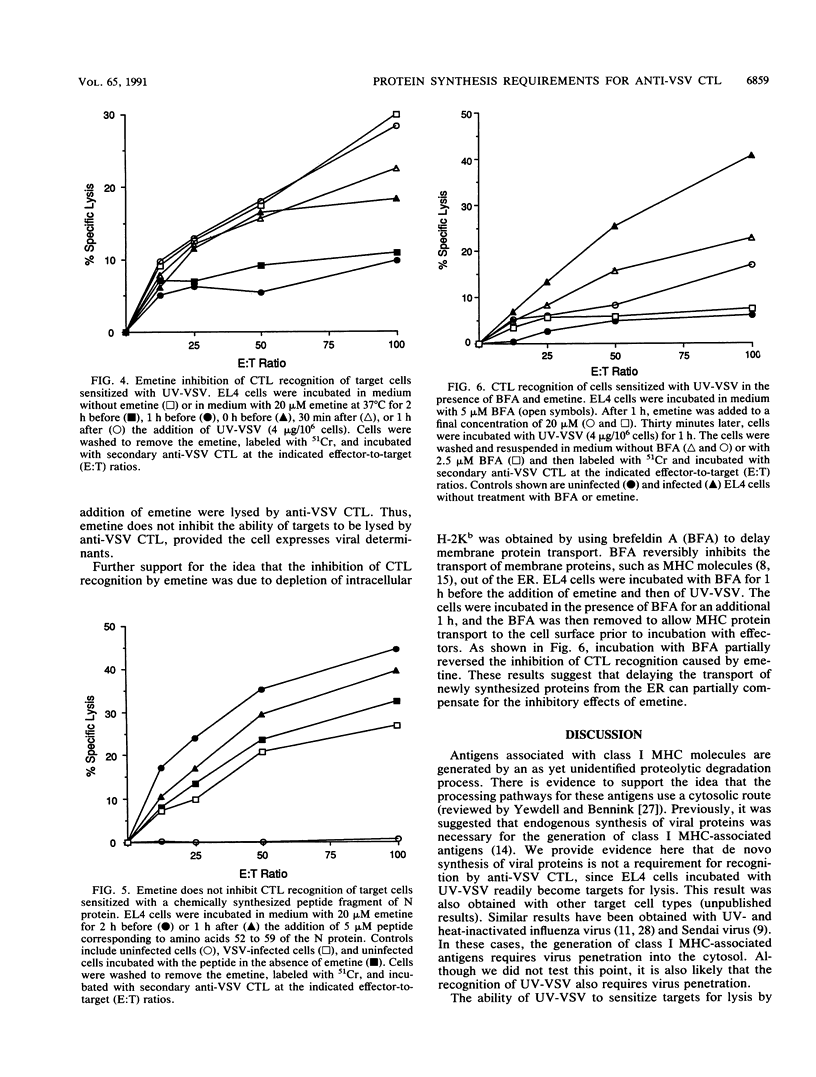
The requirements for viral and host protein synthesis in the generation of target antigens for cytotoxic T lymphocytes (CTL) was evaluated by using vesicular stomatitis virus (VSV) inactivated by UV irradiation (UV-VSV). EL4 target cells incubated with UV-VSV were recognized and lysed by anti-VSV CTL, indicating that de novo synthesis of viral proteins was not required for the generation of antigens recognized by antiviral CTL. Anti-VSV CTL from H-2b mice primarily recognize determinants derived from the VSV N protein bound to the class I major histocompatibility complex (MHC) antigen H-2Kb. Comparison of a cloned CTL line representing this specificity and a heterogeneous population of anti-VSV CTL showed that determinants other than that recognized by the cloned CTL were generated more efficiently from UV-VSV. By using vaccinia virus recombinants that express deletion fragments of the N protein, it was shown that these additional determinants were probably derived from VSV proteins other than the N protein. The protein synthesis inhibitor emetine was used to determine whether newly synthesized host proteins were required for antigen generation. The addition of emetine to target cells prior to or at the time of the addition of UV-VSV inhibited lysis by anti-VSV CTL. This inhibition could be due to depletion of newly synthesized MHC molecules from intracellular membranes. This hypothesis was supported by using brefeldin A to delay membrane protein transport in target cells during the time of incubation with emetine and UV-VSV, which resulted in partial reversal of the effect of emetine. These results suggest that newly synthesized class I MHC molecules are required for the generation of antigens recognized by anti-VSV CTL.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Hansen T. H., Cullen S. E., Lee D. R. Slower processing, weaker beta 2-M association, and lower surface expression of H-2Ld are influenced by its amino terminus. J Immunol. 1986 Aug 1;137(3):916–923. [PubMed] [Google Scholar]
- Bowman M. R., Lyles D. S., Parce J. W. Possible mechanisms by which the H-2Kbm3 mutation may decrease cytotoxic T-lymphocyte recognition of vesicular stomatitis virus nucleoprotein antigen. J Virol. 1987 Jun;61(6):1992–1998. doi: 10.1128/jvi.61.6.1992-1998.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Both G. W., Boyle D. B. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur J Immunol. 1986 Dec;16(12):1479–1487. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Yewdell J. W., Eisenlohr L. C., Johnson P. R., Bennink J. R. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990 Feb 9;247(4943):715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- Gething M., Koszinowski U., Waterfield M. Fusion of Sendai virus with the target cell membrane is required for T cell cytotoxicity. Nature. 1978 Aug 17;274(5672):689–691. doi: 10.1038/274689a0. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Mitsugi K. Sparsomycin, an inhibitor of aminoacyl transfer to polypeptide. Biochem Biophys Res Commun. 1966 May 25;23(4):453–459. [PubMed] [Google Scholar]
- Hosaka Y., Sasao F., Yamanaka K., Bennink J. R., Yewdell J. W. Recognition of noninfectious influenza virus by class I-restricted murine cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):606–610. [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990 Aug 24;249(4971):918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- Sheil J. M., Bevan M. J., Lefrancois L. Characterization of dual-reactive H-2Kb-restricted anti-vesicular stomatitus virus and alloreactive cytotoxic T cells. J Immunol. 1987 Jun 1;138(11):3654–3660. [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Tse D. B., Pernis B. Spontaneous internalization of Class I major histocompatibility complex molecules in T lymphoid cells. J Exp Med. 1984 Jan 1;159(1):193–207. doi: 10.1084/jem.159.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Williams D. B., Borriello F., Zeff R. A., Nathenson S. G. Intracellular transport of class I histocompatibility molecules. Influence of protein folding on transport to the cell surface. J Biol Chem. 1988 Apr 5;263(10):4549–4560. [PubMed] [Google Scholar]
- Williams D. B., Swiedler S. J., Hart G. W. Intracellular transport of membrane glycoproteins: two closely related histocompatibility antigens differ in their rates of transit to the cell surface. J Cell Biol. 1985 Sep;101(3):725–734. doi: 10.1083/jcb.101.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]


