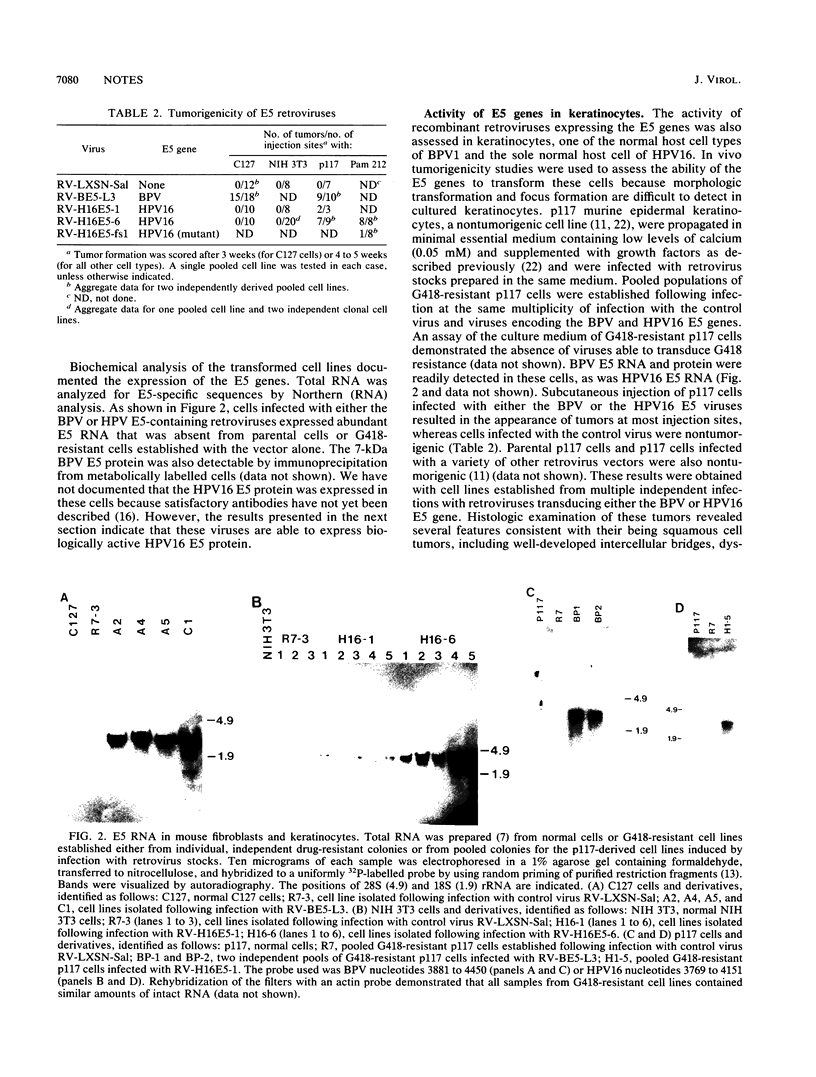
Abstract
To examine the biological properties of the bovine papillomavirus type 1 (BPV) and human papillomavirus type 16 (HPV16) E5 genes, each was cloned separately into a retroviral expression vector and helper-free recombinant viruses were generated in packaging cell lines. The BPV E5 retroviruses efficiently caused morphologic and tumorigenic transformation of cultured lines of murine fibroblasts, whereas the HPV16 E5 viruses were inactive in these assays. In contrast, infection of the p117 established line of murine epidermal keratinocytes with either the BPV or the HPV16 E5 retrovirus resulted in the generation of tumorigenic cells. Pam212 murine keratinocytes were also transformed to tumorigenicity by the HPV16 E5 gene but not by the gene carrying a frameshift mutation. These results establish that the HPV16 E5 gene is a transforming gene in cells related to its normal host epithelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bedell M. A., Jones K. H., Grossman S. R., Laimins L. A. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989 Mar;63(3):1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P., Ustav M., Sedman J., Moreno-Lopéz J., Vennström B., Pettersson U. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene. 1988 May;2(5):453–459. [PubMed] [Google Scholar]
- Bubb V., McCance D. J., Schlegel R. DNA sequence of the HPV-16 E5 ORF and the structural conservation of its encoded protein. Virology. 1988 Mar;163(1):243–246. doi: 10.1016/0042-6822(88)90259-0. [DOI] [PubMed] [Google Scholar]
- Burkhardt A., DiMaio D., Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 1987 Aug;6(8):2381–2385. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. L., Mounts P. Transforming activity of E5a protein of human papillomavirus type 6 in NIH 3T3 and C127 cells. J Virol. 1990 Jul;64(7):3226–3233. doi: 10.1128/jvi.64.7.3226-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D. Transforming activity of bovine and human papillomaviruses in cultured cells. Adv Cancer Res. 1991;56:133–159. doi: 10.1016/s0065-230x(08)60480-7. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., O'Connell J., Patskan G., Conti C., Ariza A., Slaga T. J. Malignant progression of papilloma-derived keratinocytes: differential effects of the ras, neu, and p53 oncogenes. Mol Carcinog. 1988;1(3):171–179. doi: 10.1002/mc.2940010305. [DOI] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Finbow M. E., Andresson T., McLean P., Smith K., Bubb V., Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H(+)-ATPases. Nature. 1991 Jul 25;352(6333):347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Schlegel R. The E5 oncoprotein of bovine papillomavirus binds to a 16 kd cellular protein. EMBO J. 1990 Jan;9(1):137–145. doi: 10.1002/j.1460-2075.1990.tb08089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C. L., Galloway D. A. Identification of the E5 open reading frame of human papillomavirus type 16. J Virol. 1988 Mar;62(3):1071–1075. doi: 10.1128/jvi.62.3.1071-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke R., DiMaio D. Biological properties of the deer papillomavirus E5 gene in mouse C127 cells: growth transformation, induction of DNA synthesis, and activation of the platelet-derived growth factor receptor. J Virol. 1991 Sep;65(9):4943–4949. doi: 10.1128/jvi.65.9.4943-4949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller D. R., Viaje A., Aldaz C. M., Conti C. J., Slaga T. J. Terminal differentiation-resistant epidermal cells in mice undergoing two-stage carcinogenesis. Cancer Res. 1987 Apr 1;47(7):1935–1940. [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez J., Ahola H., Eriksson A., Bergman P., Pettersson U. Reindeer papillomavirus transforming properties correlate with a highly conserved E5 region. J Virol. 1987 Nov;61(11):3394–3400. doi: 10.1128/jvi.61.11.3394-3400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary K., DiMaio D. Open reading frames E6 and E7 of bovine papillomavirus type 1 are both required for full transformation of mouse C127 cells. J Virol. 1989 Jan;63(1):259–266. doi: 10.1128/jvi.63.1.259-266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Reiss M., DiMaio D., Zibello T. A. Bovine papillomavirus type I induces resistance to Ca+(+)-induced terminal differentiation in murine keratinocytes. Cancer Commun. 1989;1(2):75–82. doi: 10.3727/095535489820875318. [DOI] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Doniger J., DiPaolo J. A., Lowy D. R. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988 Sep;3(2):167–175. [PubMed] [Google Scholar]
- Yang Y. C., Spalholz B. A., Rabson M. S., Howley P. M. Dissociation of transforming and trans-activation functions for bovine papillomavirus type 1. Nature. 1985 Dec 12;318(6046):575–577. doi: 10.1038/318575a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]