Abstract
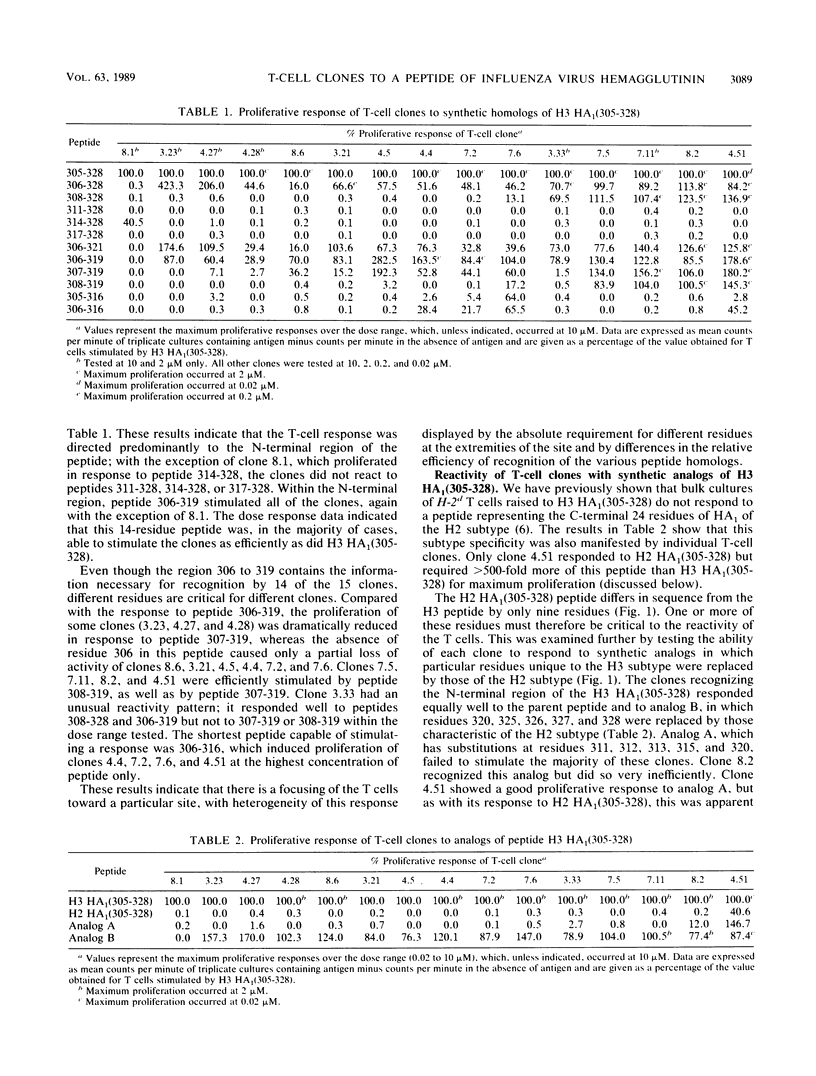
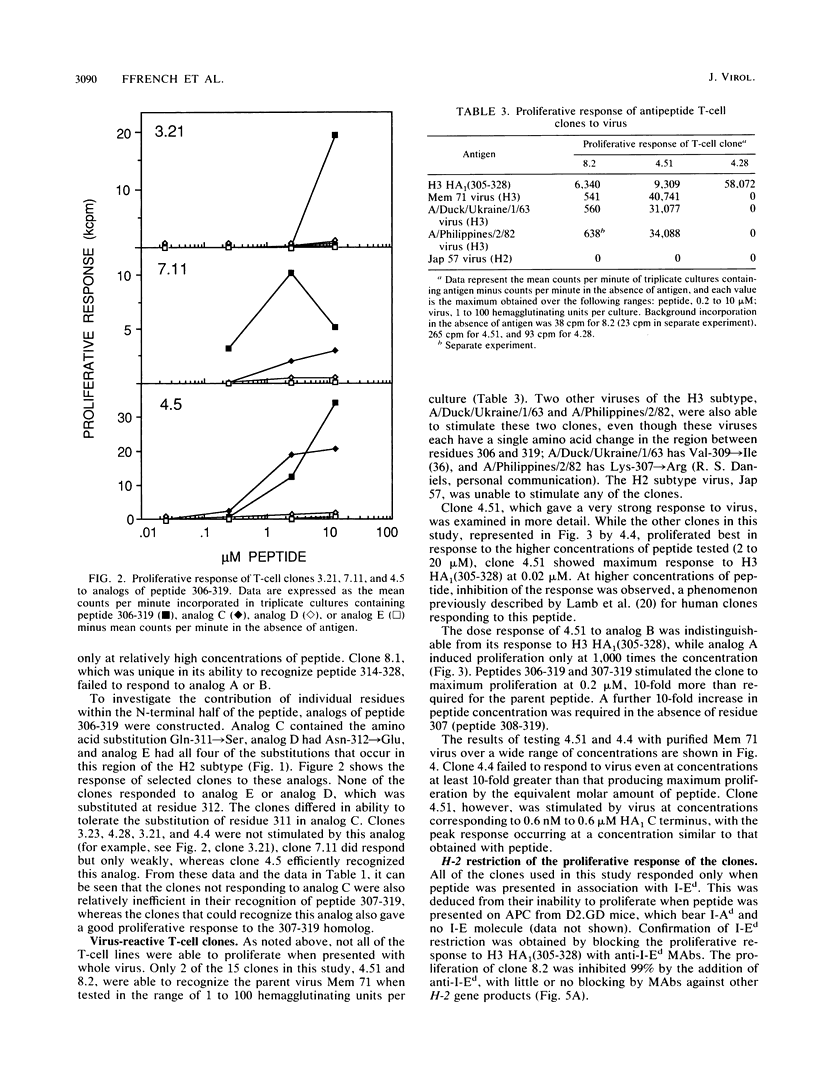
Fifteen T-cell clones were derived from BALB/c or DBA/2 mice immunized with a synthetic peptide corresponding to the C-terminal 24 residues (residues 305 to 328) of the HA1 chain of H3 subtype influenza virus hemagglutinin. All of the clones proliferated when the peptide was presented in association with I-Ed. By using shorter homologs, it was shown that the T-cell response was focused predominantly on the region at the N-terminal end of the peptide encompassed by residues 306 to 319. Individual clones recognizing this region differed in their absolute requirements for residues at the extremities of the site and also in their patterns of efficiency of recognition of shorter homologs. One particular clone defined another site of T-cell recognition within residues 314 to 328. The response of the clones to peptide analogs identified certain residues within the sites that were critical for recognition, with the substitution Gln-311----Ser having a differential effect on clones responding to the N-terminal site. Only one of the clones responded well to influenza virus itself. This clone also required relatively low concentrations of the parent peptide for optimum stimulation and was suppressed by higher concentrations. The data demonstrate striking heterogeneity in the T-cell response even to a short synthetic peptide, with different T-cell clones recognizing slightly different but overlapping areas of the molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Matsueda G. R., Haber E., Unanue E. R. Specificity of the T cell receptor: two different determinants are generated by the same peptide and the I-Ak molecule. J Immunol. 1985 Jul;135(1):368–373. [PubMed] [Google Scholar]
- Berkower I., Buckenmeyer G. K., Berzofsky J. A. Molecular mapping of a histocompatibility-restricted immunodominant T cell epitope with synthetic and natural peptides: implications for T cell antigenic structure. J Immunol. 1986 Apr 1;136(7):2498–2503. [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Ffrench R. A., Gawler J. M., Jackson D. C., Dyall-Smith M. L., Anders E. M., Tregear G. W., Duncan L., Underwood P. A., White D. O. Distinct epitopes recognized by I-Ad-restricted T-cell clones within antigenic site E on influenza virus hemagglutinin. J Virol. 1988 Jan;62(1):305–312. doi: 10.1128/jvi.62.1.305-312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. E., Katz J. M., Ffrench R. A., Anders E. M., White D. O. Characterization of subtype-specific and cross-reactive helper-T-cell clones recognizing influenza virus hemagglutinin. Cell Immunol. 1987 Oct 1;109(1):12–24. doi: 10.1016/0008-8749(87)90288-7. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Murray J. M., Anders E. M., Tang X. L., White D. O., Tregear G. W., Jackson D. C. Genetic control and fine specificity of the immune response to a synthetic peptide of influenza virus hemagglutinin. J Virol. 1988 May;62(5):1746–1752. doi: 10.1128/jvi.62.5.1746-1752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cease K. B., Berkower I., York-Jolley J., Berzofsky J. A. T cell clones specific for an amphipathic alpha-helical region of sperm whale myoglobin show differing fine specificities for synthetic peptides. A multiview/single structure interpretation of immunodominance. J Exp Med. 1986 Nov 1;164(5):1779–1784. doi: 10.1084/jem.164.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Sercarz E. E. Preferential pairing of T-B specificities in the same antigen: the concept of directional help. Vaccine. 1988 Apr;6(2):94–98. doi: 10.1016/s0264-410x(88)80006-9. [DOI] [PubMed] [Google Scholar]
- DONALD H. B., ISAACS A. Counts of influenza virus particles. J Gen Microbiol. 1954 Jun;10(3):457–464. doi: 10.1099/00221287-10-3-457. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Role of receptor-binding activity of the viral hemagglutinin molecule in the presentation of influenza virus antigens to helper T cells. J Virol. 1987 May;61(5):1375–1383. doi: 10.1128/jvi.61.5.1375-1383.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Hackett C. J., Dietzschold B., Gerhard W., Ghrist B., Knorr R., Gillessen D., Melchers F. Influenza virus site recognized by a murine helper T cell specific for H1 strains. Localization to a nine amino acid sequence in the hemagglutinin molecule. J Exp Med. 1983 Aug 1;158(2):294–302. doi: 10.1084/jem.158.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett C. J., Hurwitz J. L., Dietzschold B., Gerhard W. A synthetic decapeptide of influenza virus hemagglutinin elicits helper T cells with the same fine recognition specificities as occur in response to whole virus. J Immunol. 1985 Aug;135(2):1391–1394. [PubMed] [Google Scholar]
- Heber-Katz E., Hollosi M., Dietzschold B., Hudecz F., Fasman G. D. The T cell response to the glycoprotein D of the herpes simplex virus: the significance of antigen conformation. J Immunol. 1985 Aug;135(2):1385–1390. [PubMed] [Google Scholar]
- Hurwitz J. L., Herber-Katz E., Hackett C. J., Gerhard W. Characterization of the murine TH response to influenza virus hemagglutinin: evidence for three major specificities. J Immunol. 1984 Dec;133(6):3371–3377. [PubMed] [Google Scholar]
- Jackson D. C., Tang X. L., Brown L. E., Murray J. M., White D. O., Tregear G. W. Antigenic determinants of influenza virus hemagglutinin. XII. the epitopes of a synthetic peptide representing the C-terminus of HA1. Virology. 1986 Dec;155(2):625–632. doi: 10.1016/0042-6822(86)90222-9. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Green N. Analysis of the antigen specificity of influenza haemagglutinin-immune human T lymphocyte clones: identification of an immunodominant region for T cells. Immunology. 1983 Dec;50(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F., Clarke J. A., Miller A., Sercarz E. E., Shastri N. A limited region within hen egg-white lysozyme serves as the focus for a diversity of T cell clones. J Immunol. 1984 Oct;133(4):2075–2078. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T-cell repertoire for antigen and MHC. Immunol Today. 1988 Oct;9(10):308–315. doi: 10.1016/0167-5699(88)91324-2. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. H., Burt D. S., Skehel J. J., Thomas D. B. Fine specificity of murine class II-restricted T cell clones for synthetic peptides of influenza virus hemagglutinin. Heterogeneity of antigen interaction with the T cell and the Ia molecule. J Immunol. 1988 Jun 15;140(12):4083–4090. [PubMed] [Google Scholar]
- Mills K. H., Skehel J. J., Thomas D. B. Extensive diversity in the recognition of influenza virus hemagglutinin by murine T helper clones. J Exp Med. 1986 Jun 1;163(6):1477–1490. doi: 10.1084/jem.163.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestorowicz A., Tregear G. W., Southwell C. N., Martyn J., Murray J. M., White D. O., Jackson D. C. Antibodies elicited by influenza virus hemagglutinin fail to bind to synthetic peptides representing putative antigenic sites. Mol Immunol. 1985 Feb;22(2):145–154. doi: 10.1016/s0161-5890(85)80008-0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rothbard J. B., Lechler R. I., Howland K., Bal V., Eckels D. D., Sekaly R., Long E. O., Taylor W. R., Lamb J. R. Structural model of HLA-DR1 restricted T cell antigen recognition. Cell. 1988 Feb 26;52(4):515–523. doi: 10.1016/0092-8674(88)90464-3. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs P. G., Geysen H. M., Jackson D. C., Brown L. E., Tang X. L., White D. O. Epitopes of an influenza viral peptide recognized by antibody at single amino acid resolution. J Immunol. 1988 Jan 15;140(2):611–616. [PubMed] [Google Scholar]
- Schwartz R. H., Fox B. S., Fraga E., Chen C., Singh B. The T lymphocyte response to cytochrome c. V. Determination of the minimal peptide size required for stimulation of T cell clones and assessment of the contribution of each residue beyond this size to antigenic potency. J Immunol. 1985 Oct;135(4):2598–2608. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Suzuki G., Kawase Y., Koyasu S., Yahara I., Kobayashi Y., Schwartz R. H. Antigen-induced suppression of the proliferative response of T cell clones. J Immunol. 1988 Mar 1;140(5):1359–1365. [PubMed] [Google Scholar]
- Tang X. L., Tregear G. W., White D. O., Jackson D. C. Minimum requirements for immunogenic and antigenic activities of homologs of a synthetic peptide of influenza virus hemagglutinin. J Virol. 1988 Dec;62(12):4745–4751. doi: 10.1128/jvi.62.12.4745-4751.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. W. Structure of the influenza virus hemagglutinin. Curr Top Microbiol Immunol. 1981;94-95:1–74. doi: 10.1007/978-3-642-68120-2_1. [DOI] [PubMed] [Google Scholar]


