Abstract
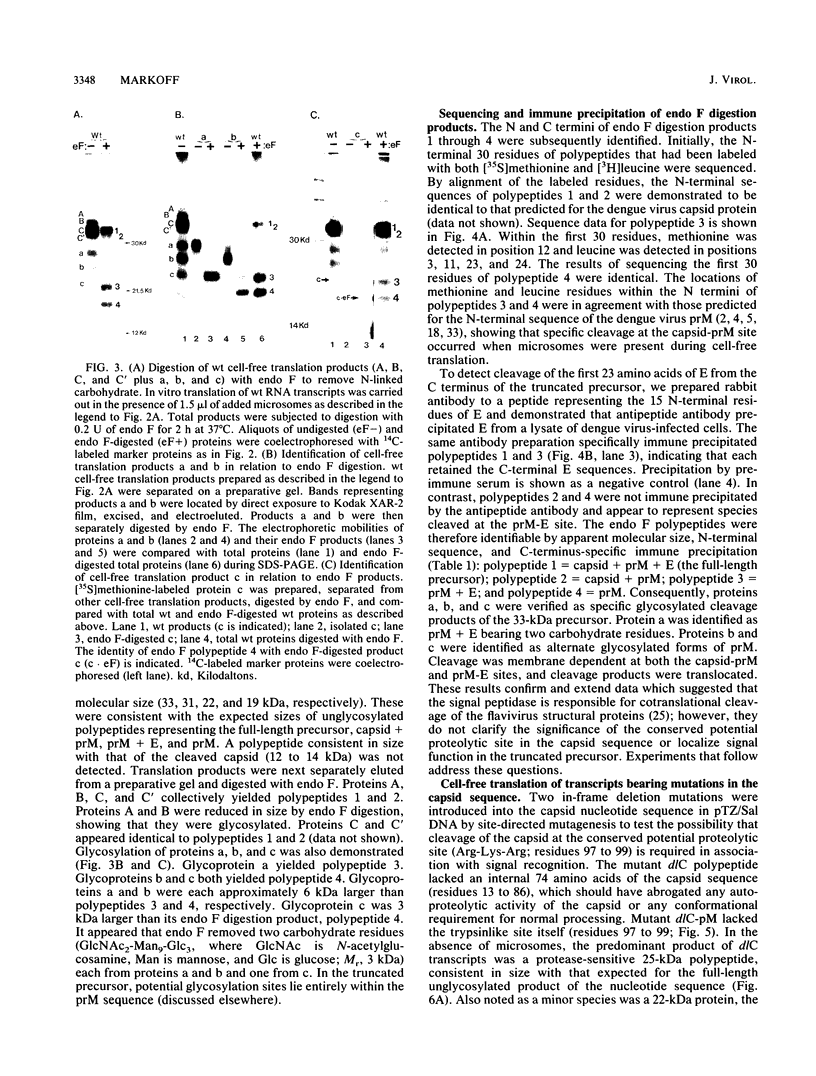
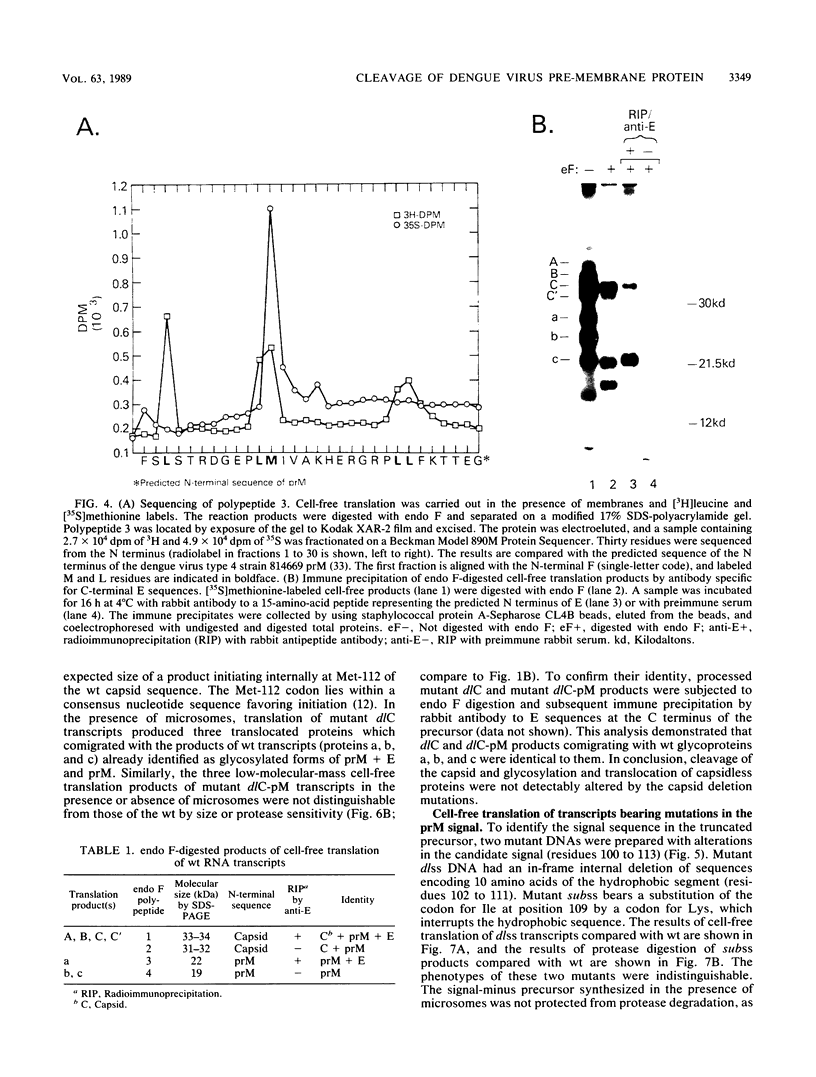
Processing of dengue virus structural proteins was assessed in vitro. RNA transcripts for cell-free translation were prepared from cloned DNA (dengue virus type 4, strain 814669 genome) encoding capsid, pre-membrane (prM), and the first 23 amino acids of envelope (E). Processing of a 33-kilodalton precursor polypeptide encoded by wild-type RNA transcripts occurred only in the presence of added microsomal membranes. Under these conditions, cleavage at the capsid-prM and prM-E sites and glycosylation of prM occurred in association with translocation. Amino acid sequence analysis confirmed that translation initiated at the predicted N terminus of the capsid and that capsid-prM cleavage occurred at the predicted site for the action of signal peptidase following a candidate signal sequence (hydrophobic residues 100 to 113) in the dengue virus precursor. Mutations were introduced into the dengue virus DNA template by site-directed mutagenesis, altering nucleotide sequences encoding the capsid and the candidate signal for prM. The phenotypes of the mutants were deduced by analysis of the products of cell-free translation of the respective RNA transcripts. The resulting observations confirmed that cleavage at the capsid-prM and prM-E sites is effected entirely by signal peptidase and that the candidate signal is required for translocation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bause E., Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981 Jun 1;195(3):639–644. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. R., Kinney R. M., Trent D. W., Lenches E. M., Dalgarno L., Strauss J. H. Amino-terminal amino acid sequences of structural proteins of three flaviviruses. Virology. 1985 May;143(1):224–229. doi: 10.1016/0042-6822(85)90110-2. [DOI] [PubMed] [Google Scholar]
- Boulton R. W., Westaway E. G. Comparisons of togaviruses: Sindbis virus (group A) and Kunjin virus (group B). Virology. 1972 Jul;49(1):283–289. doi: 10.1016/s0042-6822(72)80029-1. [DOI] [PubMed] [Google Scholar]
- Castle E., Nowak T., Leidner U., Wengler G., Wengler G. Sequence analysis of the viral core protein and the membrane-associated proteins V1 and NV2 of the flavivirus West Nile virus and of the genome sequence for these proteins. Virology. 1985 Sep;145(2):227–236. doi: 10.1016/0042-6822(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Coia G., Parker M. D., Speight G., Byrne M. E., Westaway E. G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988 Jan;69(Pt 1):1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Trent D. W., Strauss J. H., Rice C. M. Partial nucleotide sequence of the Murray Valley encephalitis virus genome. Comparison of the encoded polypeptides with yellow fever virus structural and non-structural proteins. J Mol Biol. 1986 Feb 5;187(3):309–323. doi: 10.1016/0022-2836(86)90435-3. [DOI] [PubMed] [Google Scholar]
- Deubel V., Kinney R. M., Trent D. W. Nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue type 2 virus, Jamaica genotype. Virology. 1986 Dec;155(2):365–377. doi: 10.1016/0042-6822(86)90200-x. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y. S., Galler R., Hunkapiller T., Dalrymple J. M., Strauss J. H., Strauss E. G. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology. 1988 Jan;162(1):167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Hase T., Summers P. L., Eckels K. H., Baze W. B. An electron and immunoelectron microscopic study of dengue-2 virus infection of cultured mosquito cells: maturation events. Arch Virol. 1987;92(3-4):273–291. doi: 10.1007/BF01317484. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lyapustin V. N., Svitkin YuV, Ugarova TYu, Lashkevich V. A., Agol V. I. A tentative model of formation of structural proteins of tick-borne encephalitis virus (flavivirus). FEBS Lett. 1986 May 12;200(2):314–316. doi: 10.1016/0014-5793(86)81159-0. [DOI] [PubMed] [Google Scholar]
- Mackow E., Makino Y., Zhao B. T., Zhang Y. M., Markoff L., Buckler-White A., Guiler M., Chanock R., Lai C. J. The nucleotide sequence of dengue type 4 virus: analysis of genes coding for nonstructural proteins. Virology. 1987 Aug;159(2):217–228. doi: 10.1016/0042-6822(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Mason P. W., McAda P. C., Mason T. L., Fournier M. J. Sequence of the dengue-1 virus genome in the region encoding the three structural proteins and the major nonstructural protein NS1. Virology. 1987 Nov;161(1):262–267. doi: 10.1016/0042-6822(87)90196-6. [DOI] [PubMed] [Google Scholar]
- McAda P. C., Mason P. W., Schmaljohn C. S., Dalrymple J. M., Mason T. L., Fournier M. J. Partial nucleotide sequence of the Japanese encephalitis virus genome. Virology. 1987 Jun;158(2):348–360. doi: 10.1016/0042-6822(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A., Heinrich R., Walter P., Schulmeister T. Mathematical modeling of the effects of the signal recognition particle on translation and translocation of proteins across the endoplasmic reticulum membrane. J Mol Biol. 1987 Jun 5;195(3):621–636. doi: 10.1016/0022-2836(87)90186-0. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967 Aug;99(2):291–296. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight G., Coia G., Parker M. D., Westaway E. G. Gene mapping and positive identification of the non-structural proteins NS2A, NS2B, NS3, NS4B and NS5 of the flavivirus Kunjin and their cleavage sites. J Gen Virol. 1988 Jan;69(Pt 1):23–34. doi: 10.1099/0022-1317-69-1-23. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P. Amino acid sequence of the heavy chain of bovine protein C. J Biol Chem. 1982 Oct 25;257(20):12180–12190. [PubMed] [Google Scholar]
- Svitkin Y. V., Ugarova T. Y., Chernovskaya T. V., Lyapustin V. N., Lashkevich V. A., Agol V. I. Translation of tick-borne encephalitis virus (flavivirus) genome in vitro: synthesis of two structural polypeptides. Virology. 1981 Apr 15;110(1):26–34. doi: 10.1016/0042-6822(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Kinney R. M., Johnson B. J., Vorndam A. V., Grant J. A., Deubel V., Rice C. M., Hahn C. Partial nucleotide sequence of St. Louis encephalitis virus RNA: structural proteins, NS1, ns2a, and ns2b. Virology. 1987 Feb;156(2):293–304. doi: 10.1016/0042-6822(87)90409-0. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Flavivirus replication strategy. Adv Virus Res. 1987;33:45–90. doi: 10.1016/s0065-3527(08)60316-4. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology. 1973 Feb;51(2):454–465. doi: 10.1016/0042-6822(73)90444-3. [DOI] [PubMed] [Google Scholar]
- Winkler G., Heinz F. X., Kunz C. Studies on the glycosylation of flavivirus E proteins and the role of carbohydrate in antigenic structure. Virology. 1987 Aug;159(2):237–243. doi: 10.1016/0042-6822(87)90460-0. [DOI] [PubMed] [Google Scholar]
- Wright P. J., Warr H. M., Westaway E. G. Synthesis of glycoproteins in cells infected by the flavivirus Kunjin. Virology. 1981 Mar;109(2):418–427. doi: 10.1016/0042-6822(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Zhao B. T., Prince G., Horswood R., Eckels K., Summers P., Chanock R., Lai C. J. Expression of dengue virus structural proteins and nonstructural protein NS1 by a recombinant vaccinia virus. J Virol. 1987 Dec;61(12):4019–4022. doi: 10.1128/jvi.61.12.4019-4022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Mackow E., Buckler-White A., Markoff L., Chanock R. M., Lai C. J., Makino Y. Cloning full-length dengue type 4 viral DNA sequences: analysis of genes coding for structural proteins. Virology. 1986 Nov;155(1):77–88. doi: 10.1016/0042-6822(86)90169-8. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]