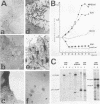
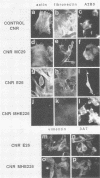
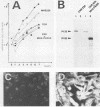
Abstract
Several studies have shown that full transformation of primary rodent fibroblasts can be achieved in vitro through the cooperation of two oncogenes (usually one nuclear and one cytoplasmic) classified on the basis of different complementation groups. We have shown previously that cooperation between v-mil (cytoplasmic, serine-threonine kinase product), and v-myc (nuclear, DNA-binding product) is required to transform 7-day-old chicken neuroretina cells, which in usual culture medium do not rapidly proliferate. v-mil induces sustained growth of chicken neuroretina cells without transformation; v-myc fails to stimulate the proliferation of chicken neuroretina cells but is required to achieve transformation of the proliferating cells. Here, we present results indicating that the P135gag-myb-ets nuclear protein of avian erythroblastosis virus E26 is able to induce proliferation but not transformation of chicken neuroretina cells. v-myc is required in addition to P135gag-myb-ets to achieve chicken neuroretina cell transformation. In contrast, we found that the P135gag-myb-ets and P100gag-mil proteins are not able to cooperate in this system.
Full text
PDF
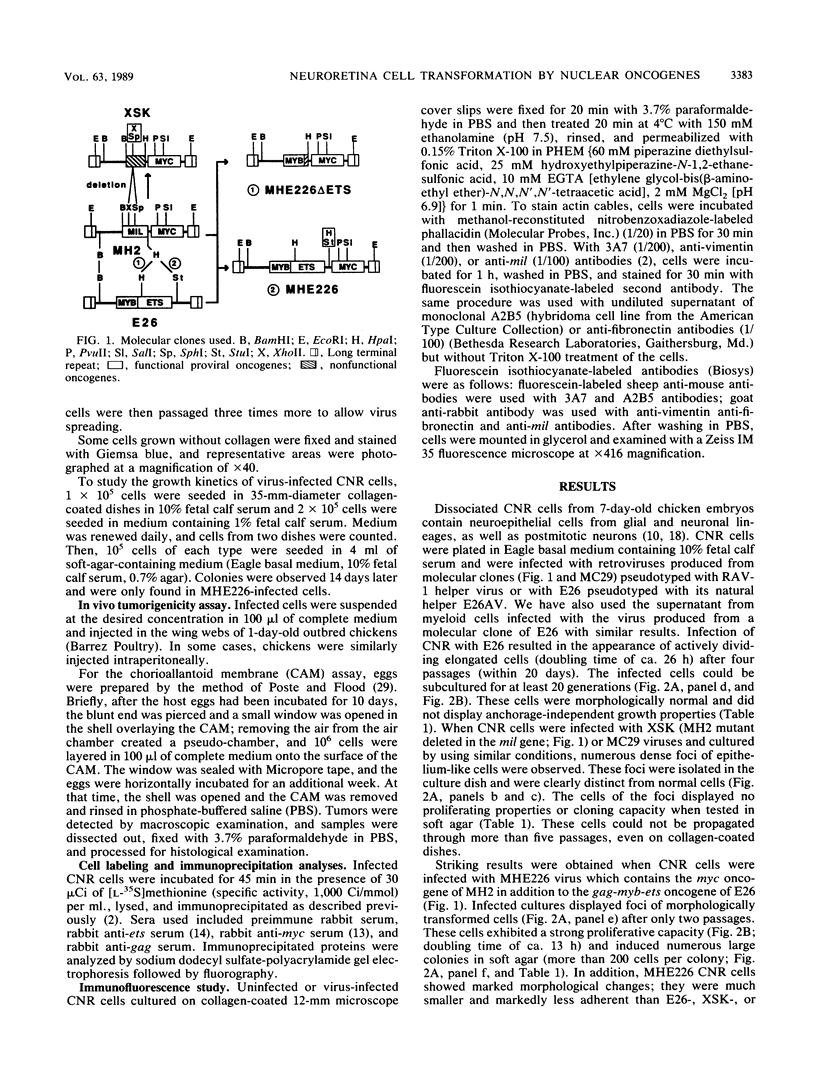



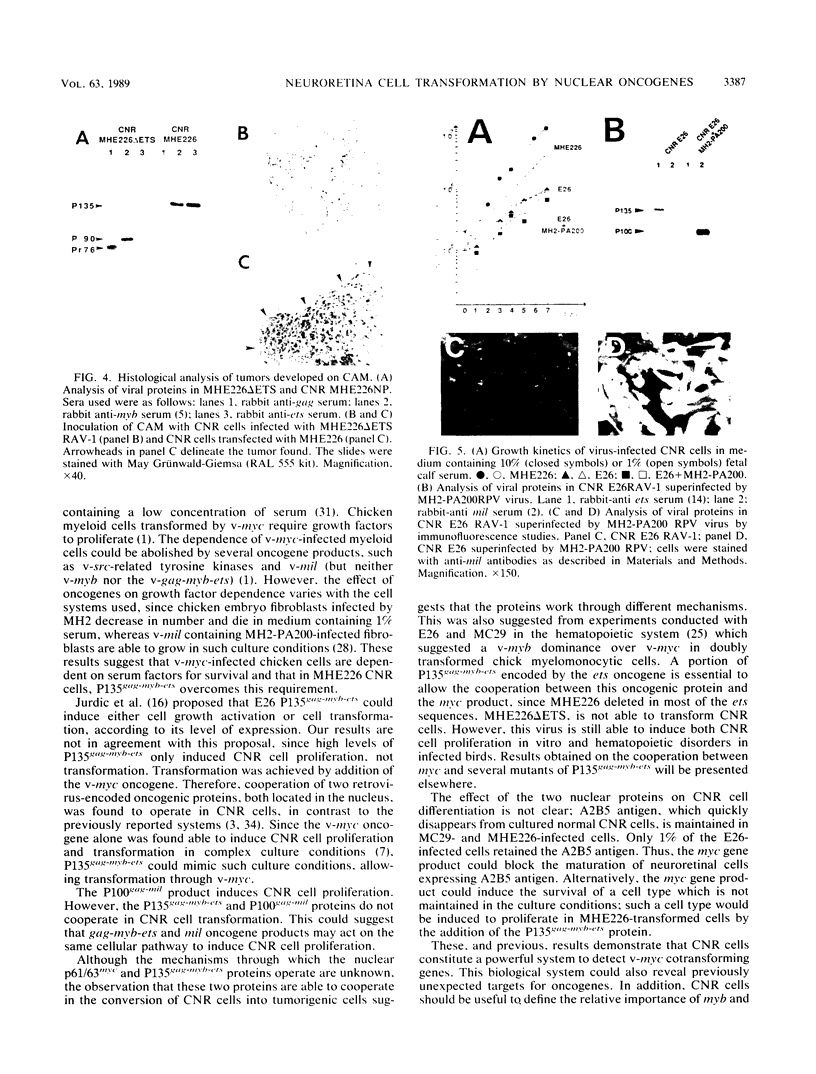

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Leutz A., Graf T. Autocrine growth induced by src-related oncogenes in transformed chicken myeloid cells. Cell. 1984 Dec;39(3 Pt 2):439–445. doi: 10.1016/0092-8674(84)90451-3. [DOI] [PubMed] [Google Scholar]
- Bechade C., Calothy G., Pessac B., Martin P., Coll J., Denhez F., Saule S., Ghysdael J., Stéhelin D. Induction of proliferation or transformation of neuroretina cells by the mil and myc viral oncogenes. Nature. 1985 Aug 8;316(6028):559–562. doi: 10.1038/316559a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Reddy E. P., Baluda M. A. Identification of the leukemogenic protein of avian myeloblastosis virus and of its normal cellular homologue. Proc Natl Acad Sci U S A. 1983 May;80(10):2834–2838. doi: 10.1073/pnas.80.10.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte T., Donner P., Pfaff E., Reis B., Greiser-Wilke I., Schaller H., Moelling K. Inhibition of DNA binding of purified p55v-myc in vitro by antibodies against bacterially expressed myc protein and a synthetic peptide. EMBO J. 1984 Aug;3(8):1919–1924. doi: 10.1002/j.1460-2075.1984.tb02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalbore P., Agostini E., Alemà S., Falcone G., Tatò F. The v-myc oncogene is sufficient to induce growth transformation of chick neuroretina cells. Nature. 1987 Mar 12;326(6109):188–190. doi: 10.1038/326188a0. [DOI] [PubMed] [Google Scholar]
- Coll J., Dozier C., Saule S., Henry C., Quatannens B., Debuire B., Stehelin D. Mapping by in vitro constructs of the P100gag-mil region, accounting for induction of chicken neuroretina cell proliferation. J Virol. 1988 Aug;62(8):2808–2816. doi: 10.1128/jvi.62.8.2808-2816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisanti-Combes P., Lorinet A. M., Girard A., Pessac B., Wasseff M., Calothy G. Expression of neuronal markers in chick and quail embryo neuroretina cultures infected with Rous sarcoma virus. Cell Differ. 1982 Jan;11(1):45–54. doi: 10.1016/0045-6039(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré F., Martin P., Begue A., Ghysdael J., Saule S., Stéhelin D. Préparation et caractérisation d'antisera spécifiques dirigés contre différents domaines polypeptidiques codés par l'oncogène c-myc humain pour étudier l'expression de ce gène introduit dans des cellules de caille ou de rat. C R Acad Sci III. 1986;303(15):633–636. [PubMed] [Google Scholar]
- Gegonne A., Leprince D., Pognonec P., Dernis D., Raes M. B., Stehelin D., Ghysdael J. The 5' extremity of the v-ets oncogene of avian leukemia virus E26 encodes amino acid sequences not derived from the major c-ets-encoded cellular proteins. Virology. 1987 Jan;156(1):177–180. doi: 10.1016/0042-6822(87)90450-8. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Jurdic P., Benchaibi M., Gandrillon O., Samarut J. Transforming and mitogenic effects of avian leukemia virus E26 on chicken hematopoietic cells and fibroblasts, respectively, correlate with level of expression of the provirus. J Virol. 1987 Oct;61(10):3058–3065. doi: 10.1128/jvi.61.10.3058-3065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Lemmon V. Monoclonal antibodies specific for glia in the chick nervous system. Brain Res. 1985 Nov;355(1):111–120. doi: 10.1016/0165-3806(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Henry C., Denhez F., Amouyel P., Bechade C., Calothy G., Debuire B., Stehelin D., Saule S. Characterization of a MH2 mutant lacking the v-myc oncogene. Virology. 1986 Sep;153(2):272–279. doi: 10.1016/0042-6822(86)90030-9. [DOI] [PubMed] [Google Scholar]
- Moelling K., Heimann B., Beimling P., Rapp U. R., Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984 Dec 6;312(5994):558–561. doi: 10.1038/312558a0. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Samarut J., Gazzolo L., Moscovici M. G. Myeloid and erythroid neoplastic responses to avian defective leukemia viruses in chickens and in quail. Virology. 1981 Sep;113(2):765–768. doi: 10.1016/0042-6822(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Jurdic P., Samarut J., Gazzolo L., Mura C. V., Moscovici C. Characterization of the hemopoietic target cells for the avian leukemia virus E26. Virology. 1983 Aug;129(1):65–78. doi: 10.1016/0042-6822(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Beug H., Graf T. v-myb dominance over v-myc in doubly transformed chick myelomonocytic cells. Cell. 1987 Oct 9;51(1):41–50. doi: 10.1016/0092-8674(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S., Vogel M. L. Fibroblast transformation parameters induced by the avian v-mil oncogene. J Virol. 1987 May;61(5):1717–1721. doi: 10.1128/jvi.61.5.1717-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Flood M. K. Cells transformed by temperature-sensitive mutants of avian sarcoma virus cause tumors in vivo at permissive and nonpermissive temperatures. Cell. 1979 Aug;17(4):789–800. doi: 10.1016/0092-8674(79)90319-2. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Saule S., Coll J., Righi M., Lagrou C., Raes M. B., Stehelin D. Two different types of transcription for the myelocytomatosis viruses MH2 and CMII. EMBO J. 1983;2(6):805–809. doi: 10.1002/j.1460-2075.1983.tb01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Fauquet M., Ziller C., Le Douarin N. M. Acetylcholine synthesis by mesencephalic neural crest cells in the process of migration in vivo. Nature. 1979 Dec 20;282(5741):853–855. doi: 10.1038/282853a0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. The action of oncogenes in the cytoplasm and nucleus. Science. 1985 Nov 15;230(4727):770–776. doi: 10.1126/science.2997917. [DOI] [PubMed] [Google Scholar]