Abstract
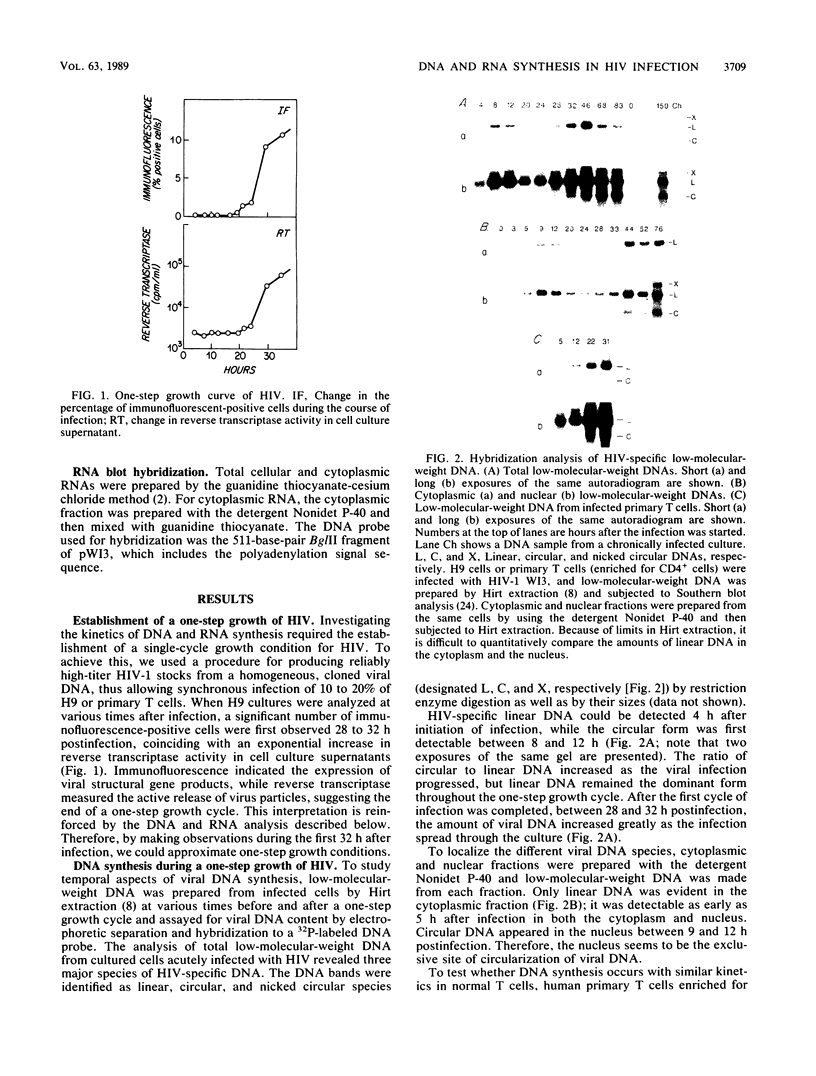
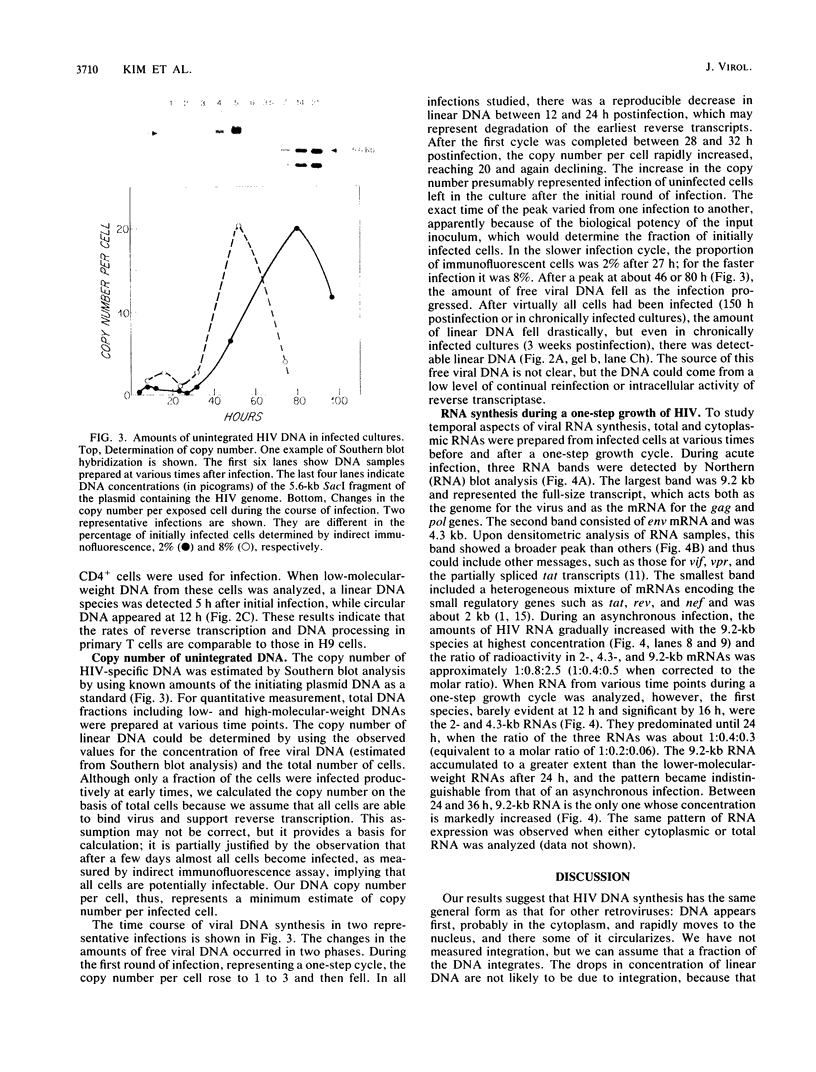
The kinetics of retroviral DNA and RNA synthesis are parameters vital to understanding viral growth, especially for human immunodeficiency virus (HIV), which encodes several of its own regulatory genes. We have established a single-cycle growth condition for HIV in H9 cells, a human CD4+ lymphocyte line. The full-length viral linear DNA is first detectable by 4 h postinfection. During a one-step growth of HIV, amounts of viral DNA gradually increase until 8 to 12 h postinfection and then decrease. The copy number of unintegrated viral DNA is not extraordinarily high even at its peak. Most strikingly, there is a temporal program of RNA accumulation: the earliest RNA is greatly enriched in the 2-kilobase subgenomic mRNA species, while the level of 9.2-kilobase RNA which is both genomic RNA and mRNA remains low until after 24 h of infection. Virus production begins at about 24 h postinfection. Thus, viral DNA synthesis is as rapid as for other retroviruses, but viral RNA synthesis involves temporal alteration in the species that accumulate, presumably as a consequence of viral regulatory genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Feng S., Holland E. C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988 Jul 14;334(6178):165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Gurley R. J., Ikeuchi K., Byrn R. A., Anderson K., Groopman J. E. CD4+ lymphocyte function with early human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1993–1997. doi: 10.1073/pnas.86.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Blum H., Scott J., Traynor B., Ventura P., Haase A. Slow virus visna: reproduction in vitro of virus from extrachromosomal DNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7212–7215. doi: 10.1073/pnas.81.22.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Schooley R. T., Rota T. R., Kaplan J. C., Flynn T., Salahuddin S. Z., Gonda M. A., Hirsch M. S. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984 Oct 26;226(4673):451–453. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Cell killing by spleen necrosis virus is correlated with a transient accumulation of spleen necrosis virus DNA. J Virol. 1979 Aug;31(2):376–388. doi: 10.1128/jvi.31.2.376-388.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Fenrick R., Cullen B. R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Mazarin V., Gourdou I., Quérat G., Sauze N., Vigne R. Genetic structure and function of an early transcript of visna virus. J Virol. 1988 Dec;62(12):4813–4818. doi: 10.1128/jvi.62.12.4813-4818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Cabradilla C. D., Benton C. V., Lasky L. A., Capon D. J. Nucleic acid structure and expression of the human AIDS/lymphadenopathy retrovirus. Nature. 1985 Feb 7;313(6002):450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Sadaie M. R., Benter T., Wong-Staal F. Site-directed mutagenesis of two trans-regulatory genes (tat-III,trs) of HIV-1. Science. 1988 Feb 19;239(4842):910–913. doi: 10.1126/science.3277284. [DOI] [PubMed] [Google Scholar]
- Sadaie M. R., Rappaport J., Benter T., Josephs S. F., Willis R., Wong-Staal F. Missense mutations in an infectious human immunodeficiency viral genome: functional mapping of tat and identification of the rev splice acceptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9224–9228. doi: 10.1073/pnas.85.23.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Hahn B. H., Arya S. K., Groopman J. E., Gallo R. C., Wong-Staal F. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science. 1984 Dec 7;226(4679):1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. Unexpectedly high levels of HIV-1 RNA and protein synthesis in a cytocidal infection. Science. 1988 Dec 16;242(4885):1554–1557. doi: 10.1126/science.3201245. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanisms of cell killing/cytopathic effects by nonhuman retroviruses. Rev Infect Dis. 1988 Mar-Apr;10(2):399–405. doi: 10.1093/clinids/10.2.399. [DOI] [PubMed] [Google Scholar]
- Vigne R., Barban V., Quérat G., Mazarin V., Gourdou I., Sauze N. Transcription of visna virus during its lytic cycle: evidence for a sequential early and late gene expression. Virology. 1987 Nov;161(1):218–227. doi: 10.1016/0042-6822(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Vujcic L. K., Shepp D. H., Klutch M., Wells M. A., Hendry R. M., Wittek A. E., Krilov L., Quinnan G. V., Jr Use of a sensitive neutralization assay to measure the prevalence of antibodies to the human immunodeficiency virus. J Infect Dis. 1988 May;157(5):1047–1050. doi: 10.1093/infdis/157.5.1047. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]