Abstract
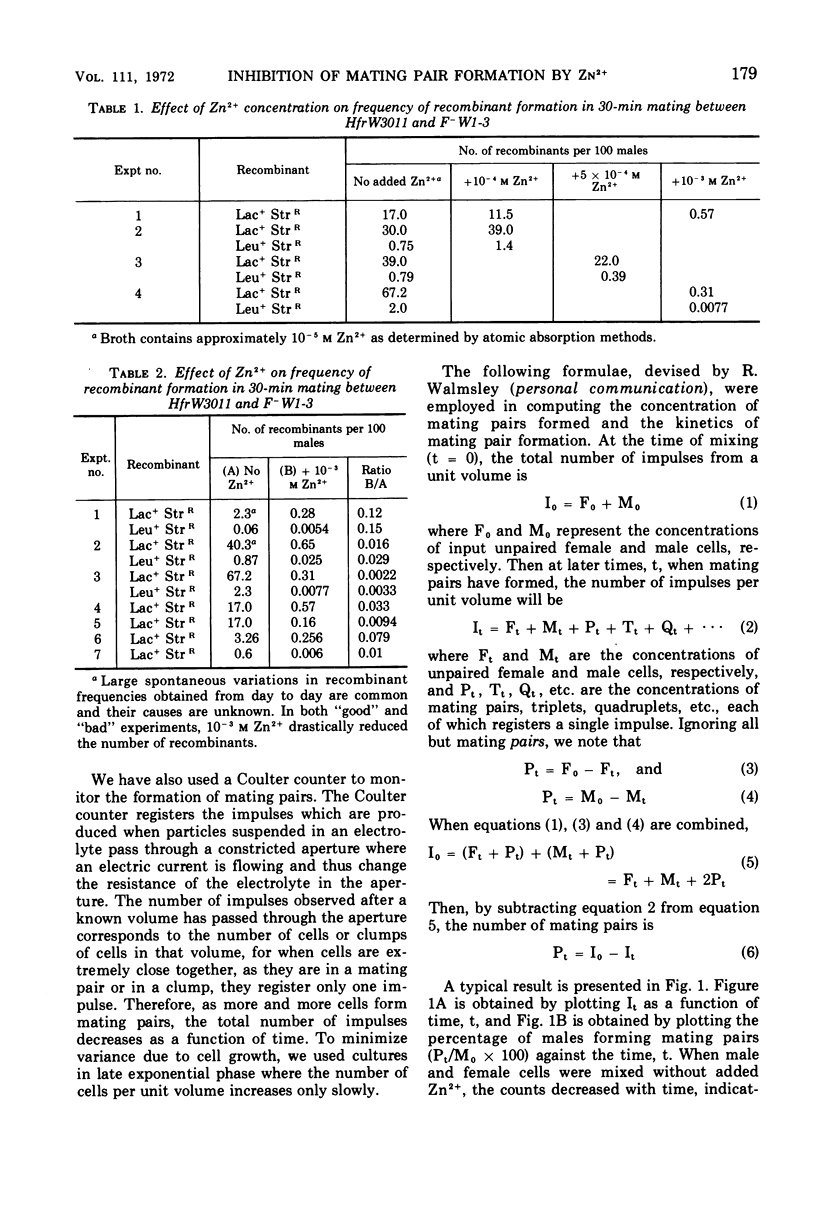
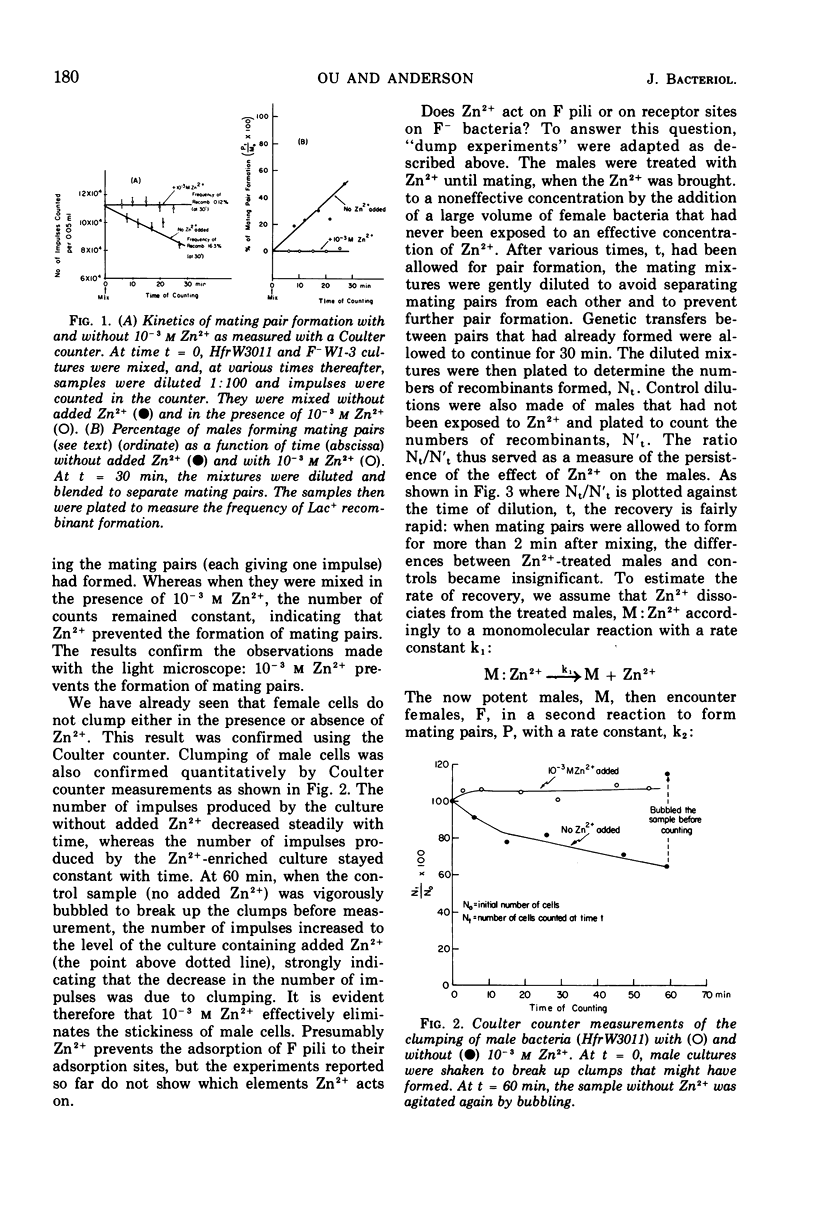
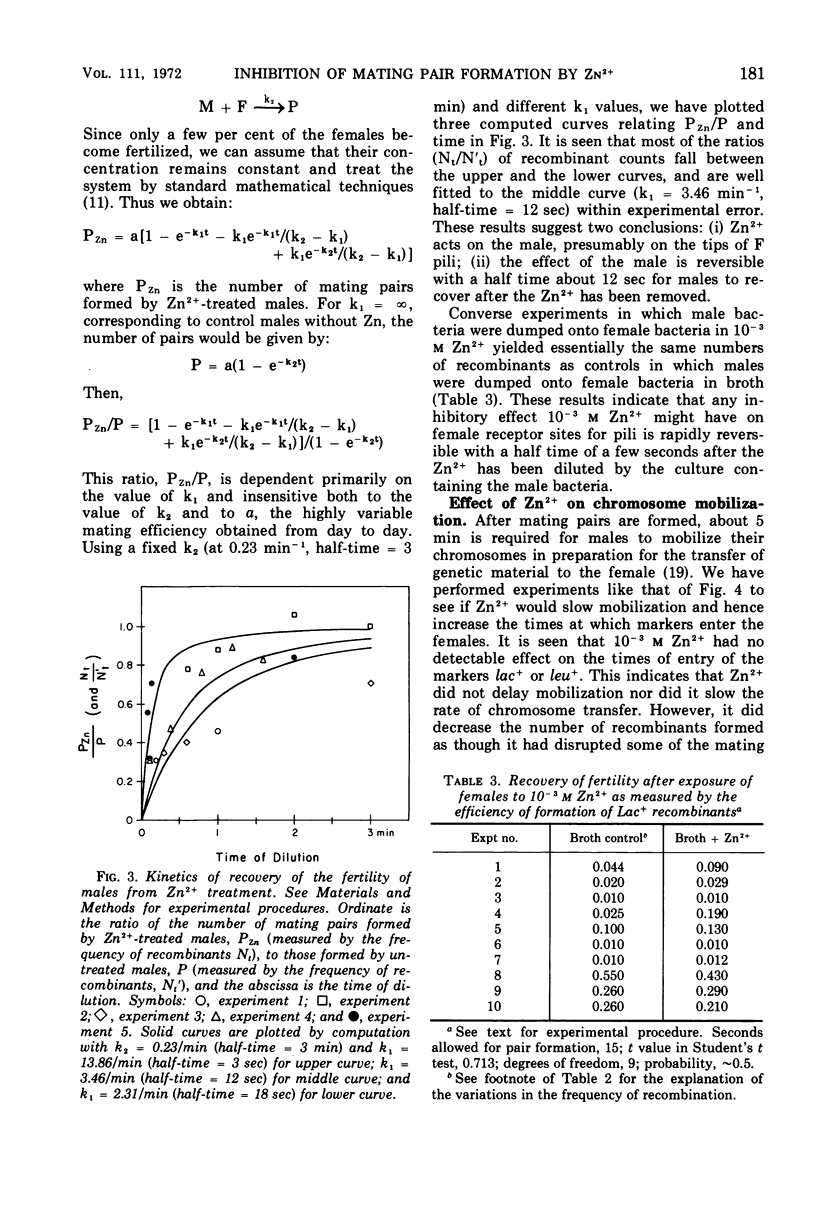
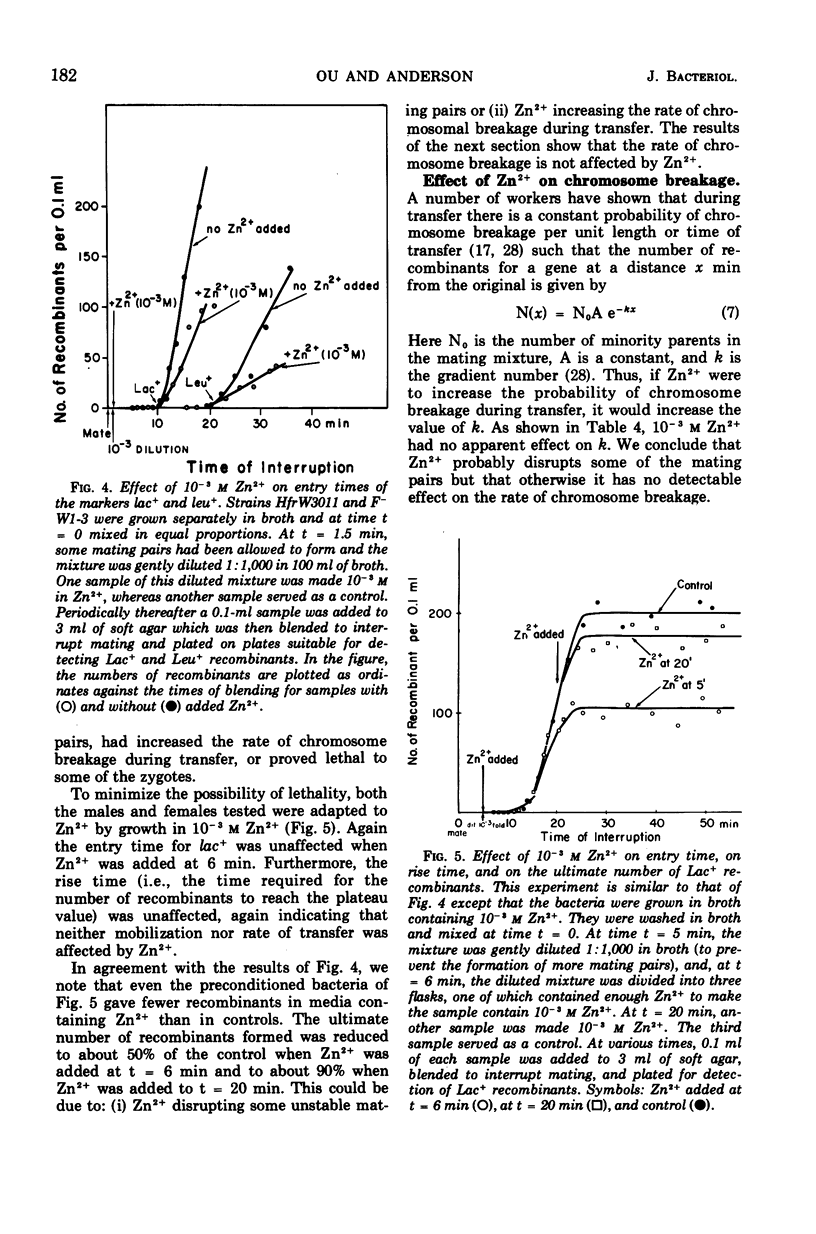
Zn2+ at 10−3m has been found to block the formation of mating pairs between Hfr and F− strains of Escherichia coli as observed both by light microscopy and by Coulter counter measurements. Kinetic studies show that Zn2+ reduces the fertility of the male and that its effect disappears within 2 min after Zn2+ has been removed from the medium. Short treatments of female cells with Zn2+ have no detectable effect on their ability to form mating pairs. Later steps in the mating process such as mobilization of the male chromosome, transfer of the chromosome to the female, or its integration into the female chromosome seem not to be affected by 10−3m Zn2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- Anderson T. F. The Activation of the Bacterial Virus T4 by l-Tryptophan. J Bacteriol. 1948 May;55(5):637–649. doi: 10.1128/jb.55.5.637-649.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Energy requirements for specific pair formation during conjugation in Escherichia coli K-12. J Bacteriol. 1967 Aug;94(2):490–492. doi: 10.1128/jb.94.2.490-492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. W. Mechanically caused damage to Hfr cells of Escherichia coli K12. Genet Res. 1966 Apr;7(2):267–271. doi: 10.1017/s0016672300009678. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Knolle P. Evidence for the identity of the mating-specific site of male cells of Escherichia coli with the receptor site of an RNA phage. Biochem Biophys Res Commun. 1967 Apr 7;27(1):81–87. doi: 10.1016/s0006-291x(67)80043-3. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Role of pili in bacterial conjugation. J Bacteriol. 1970 Jun;102(3):648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- Sneath P. H., Lederberg J. INHIBITION BY PERIODATE OF MATING IN ESCHERICHIA COLI K-12. Proc Natl Acad Sci U S A. 1961 Jan;47(1):86–90. doi: 10.1073/pnas.47.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. H. Effects of temperature, agitation, and donor strain on chromosome transfer in Escherichia coli K-12. J Bacteriol. 1968 Dec;96(6):2077–2084. doi: 10.1128/jb.96.6.2077-2084.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


