Abstract
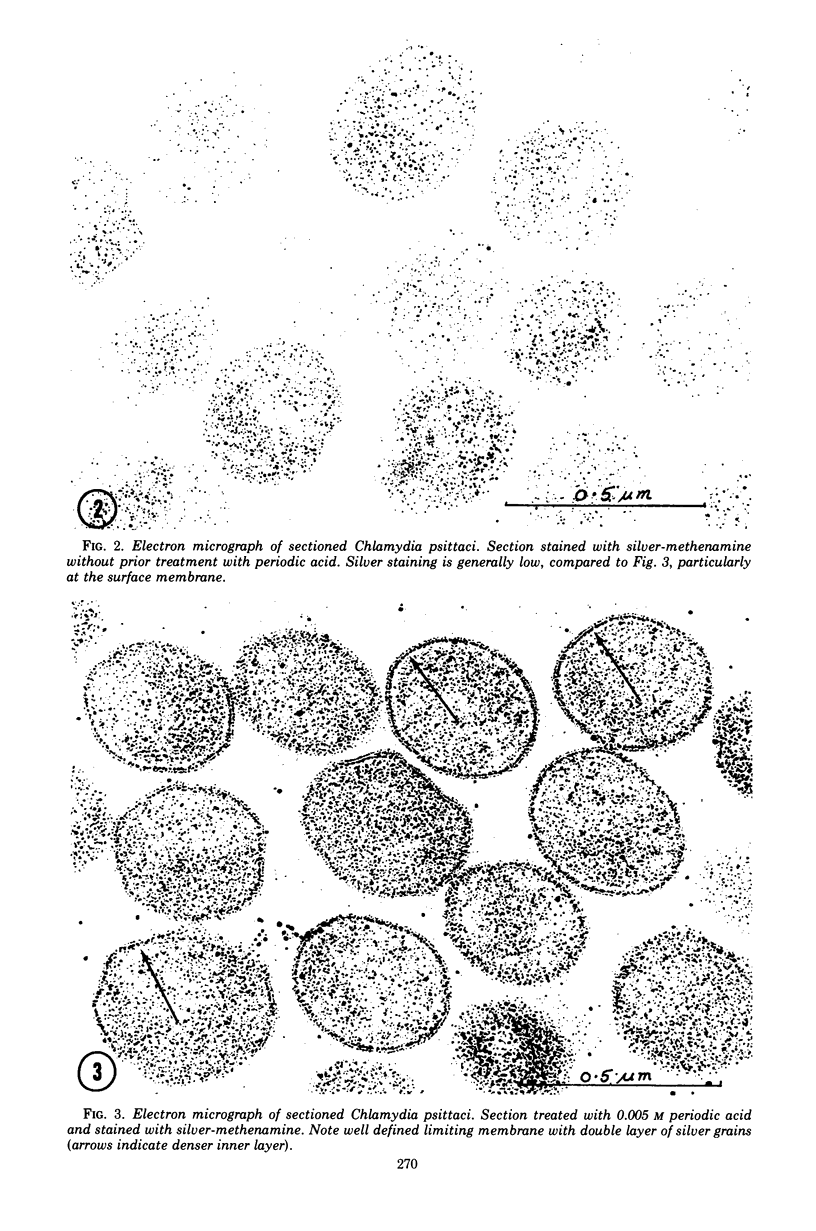
Previous serological studies have indicated that the group antigen of chlamydial organisms is composed of an acidic polysaccharide and a lipid component. The present study was undertaken in an effort to locate this polysaccharide complex by use of electron microscopy and a silver-methenamine marker. The meningopneumonitis strain of Chlamydia psittaci was propagated in HeLa-M cell culture. Organisms were purified by differential centrifugation, treatment with Genetron, and by gel filtration. After fixation and embedding, sections were obtained for electron microscopy. Sections were stained for carbohydrates with silver-methenamine. A double layer of regularly spaced silver grains of uniform size was observed at the periphery of the sectioned organisms tracing the contours of the surface membrane (cell wall). This intensity of staining was observed only when sections were oxidized with periodate prior to silver-methenamine staining. Prior treatment with 1% sodium deoxycholate resulted in a significant reduction in staining. It is considered probable that the periodate-sensitive polysaccharide found at the periphery of the organisms represents, or is a component of, the group antigen of these organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENEDICT A. A., O'BRIEN E. Antigenic studies on the psittacosis-lymphogranuloma venereum group of viruses. II. Characterization of complement-fixing antigens extracted with sodium lauryl sulfate. J Immunol. 1956 Apr;76(4):293–300. [PubMed] [Google Scholar]
- Dhir S. P., Kenny G. E., Grayston J. T. Characterization of the group antigen of Chlamydia trachomatis. Infect Immun. 1971 Dec;4(6):725–730. doi: 10.1128/iai.4.6.725-730.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M., ROSS M. R., MOULDER J. W. Species-specific antigens from the cell walls of the agents of meningopneumonitis and feline pneumonitis. J Immunol. 1961 Feb;86:123–127. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Short J. Location of bacterial polysaccharide during various phases of growth. J Bacteriol. 1969 Jun;98(3):1342–1354. doi: 10.1128/jb.98.3.1342-1354.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]