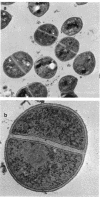
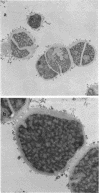
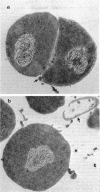
Abstract
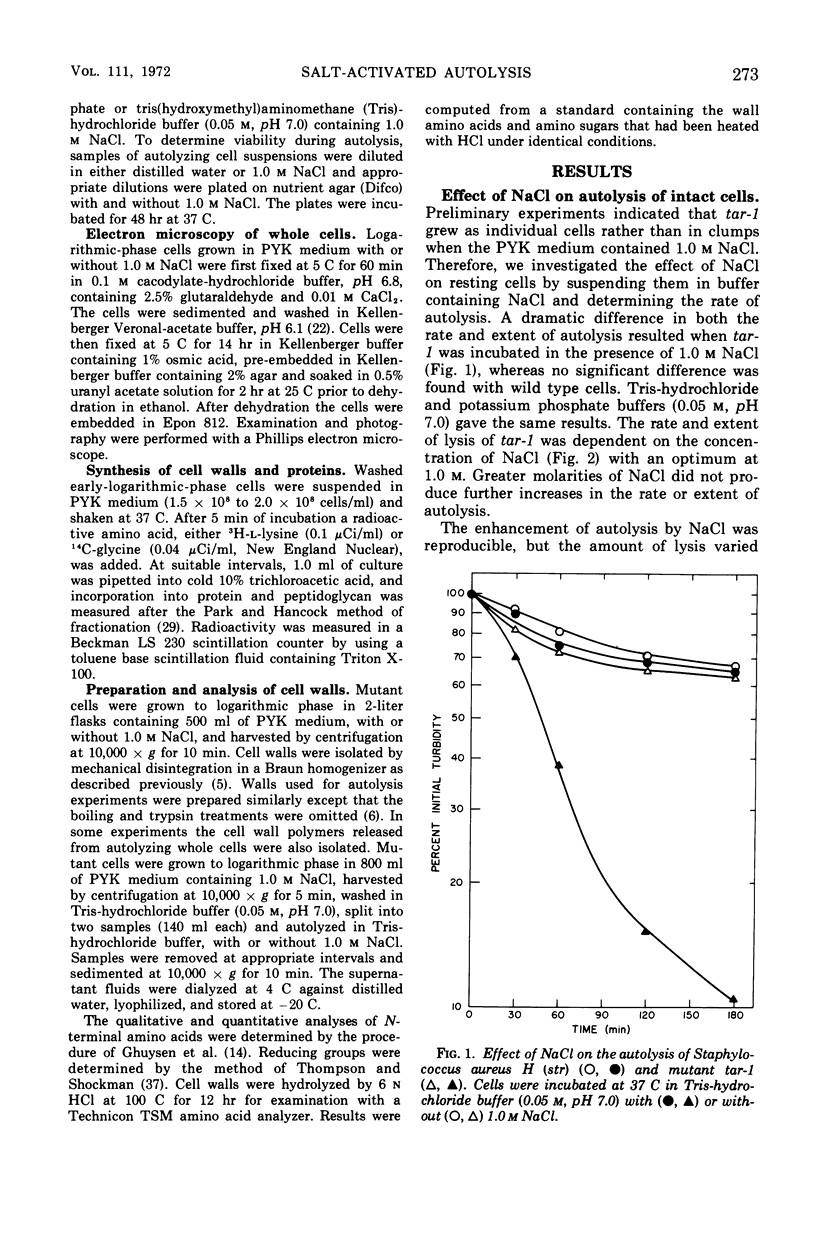
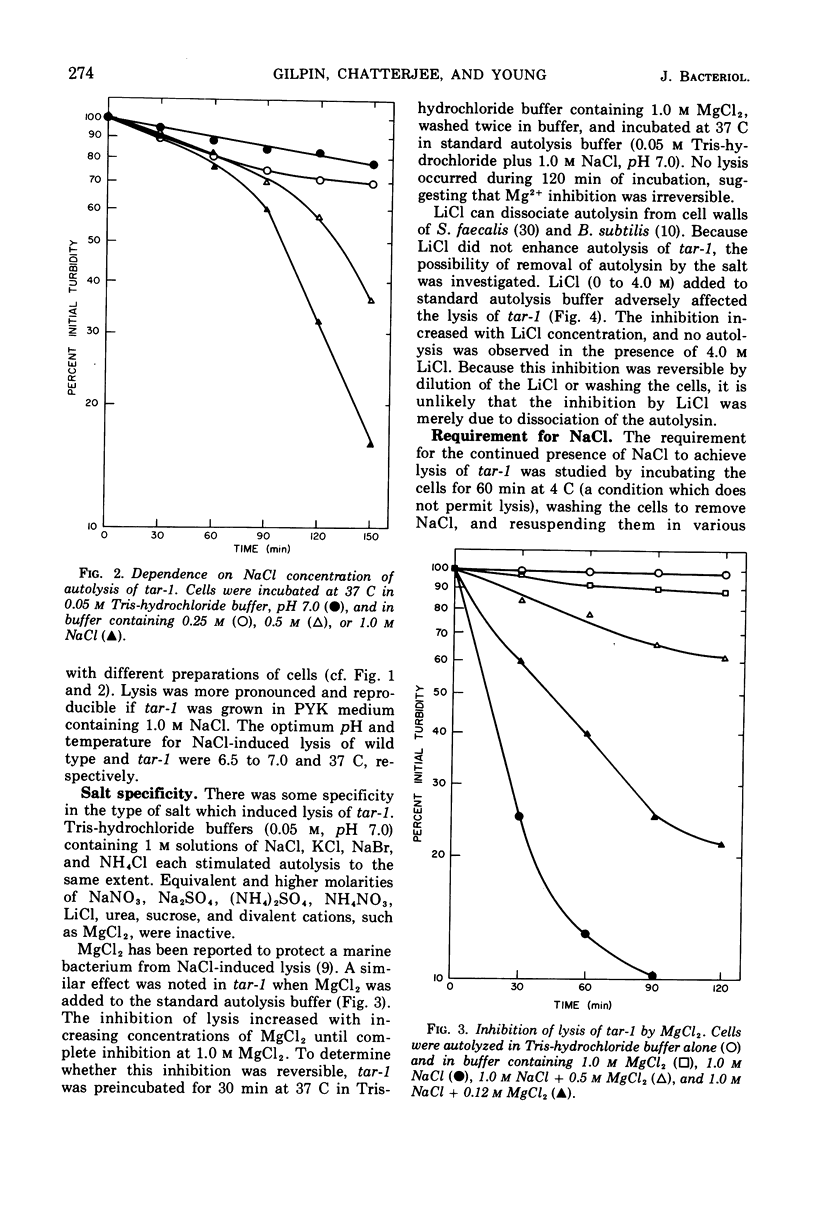
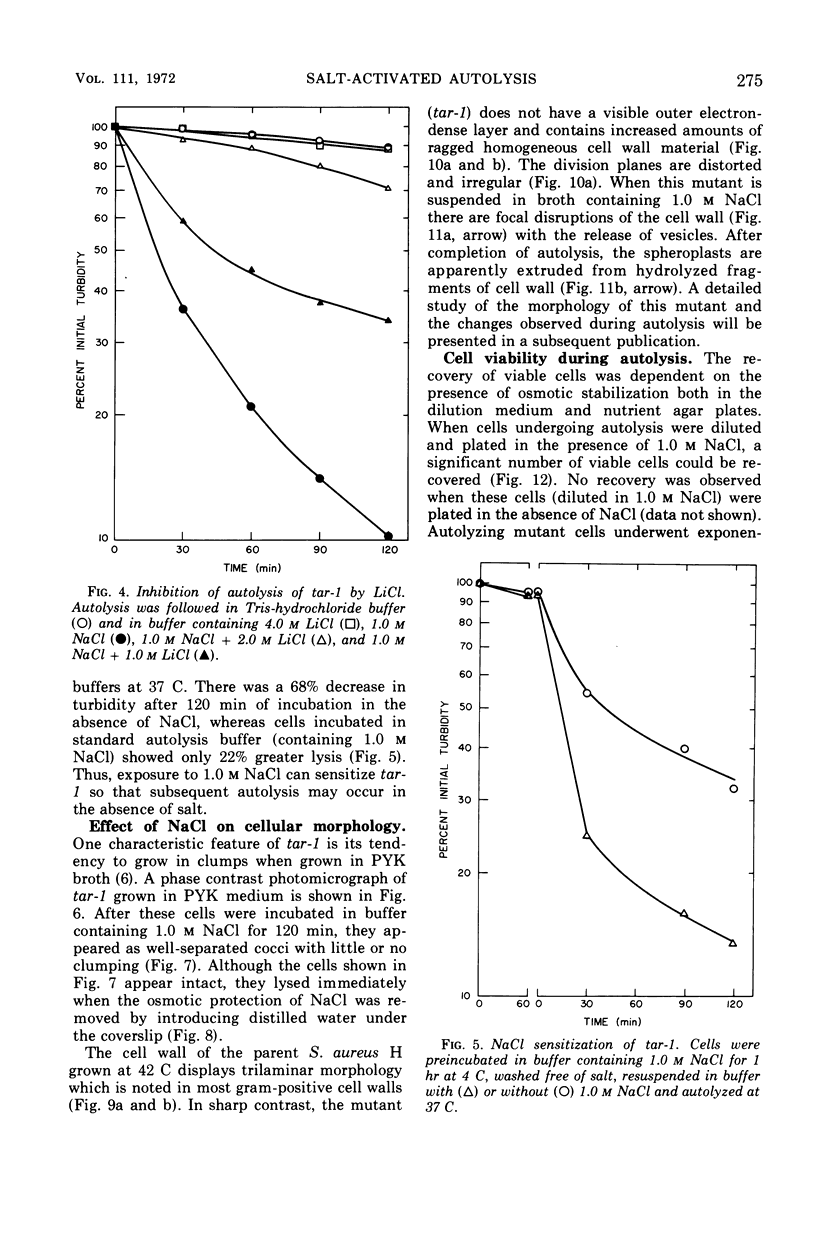
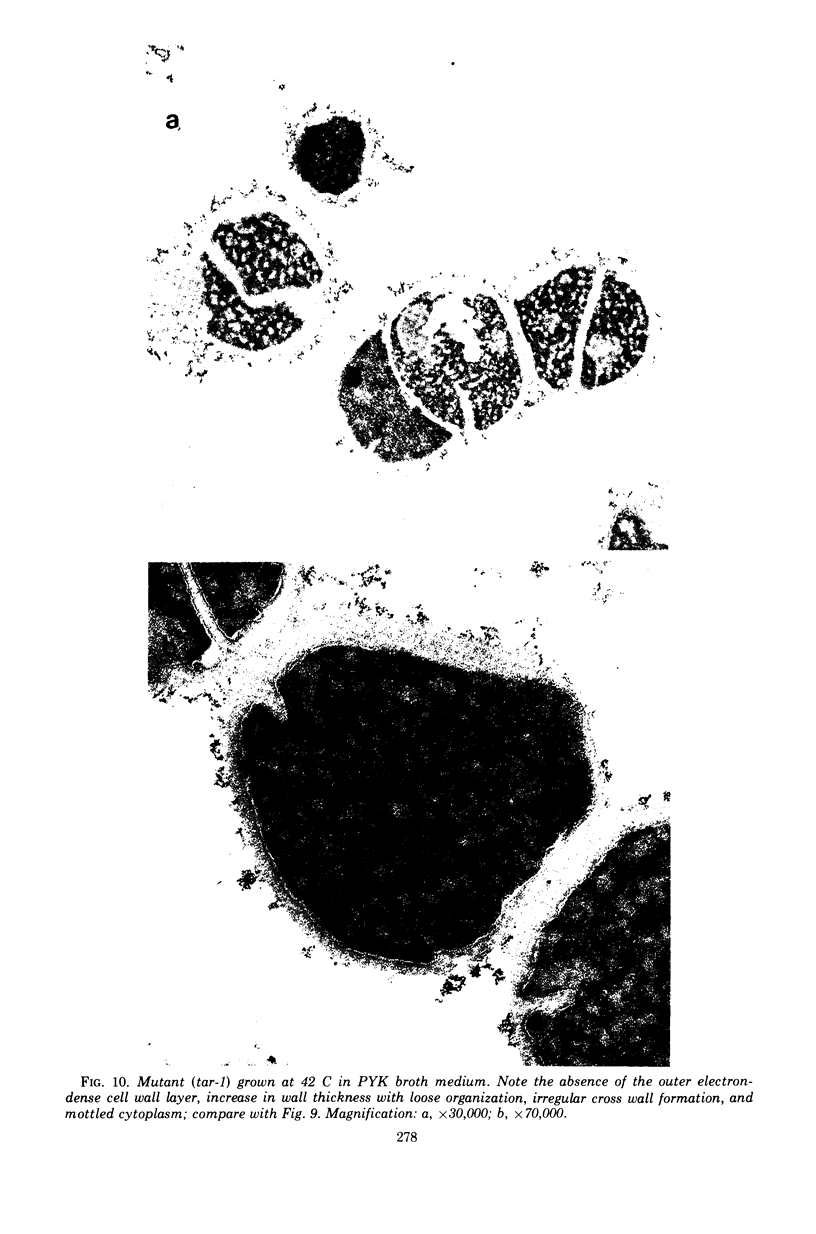
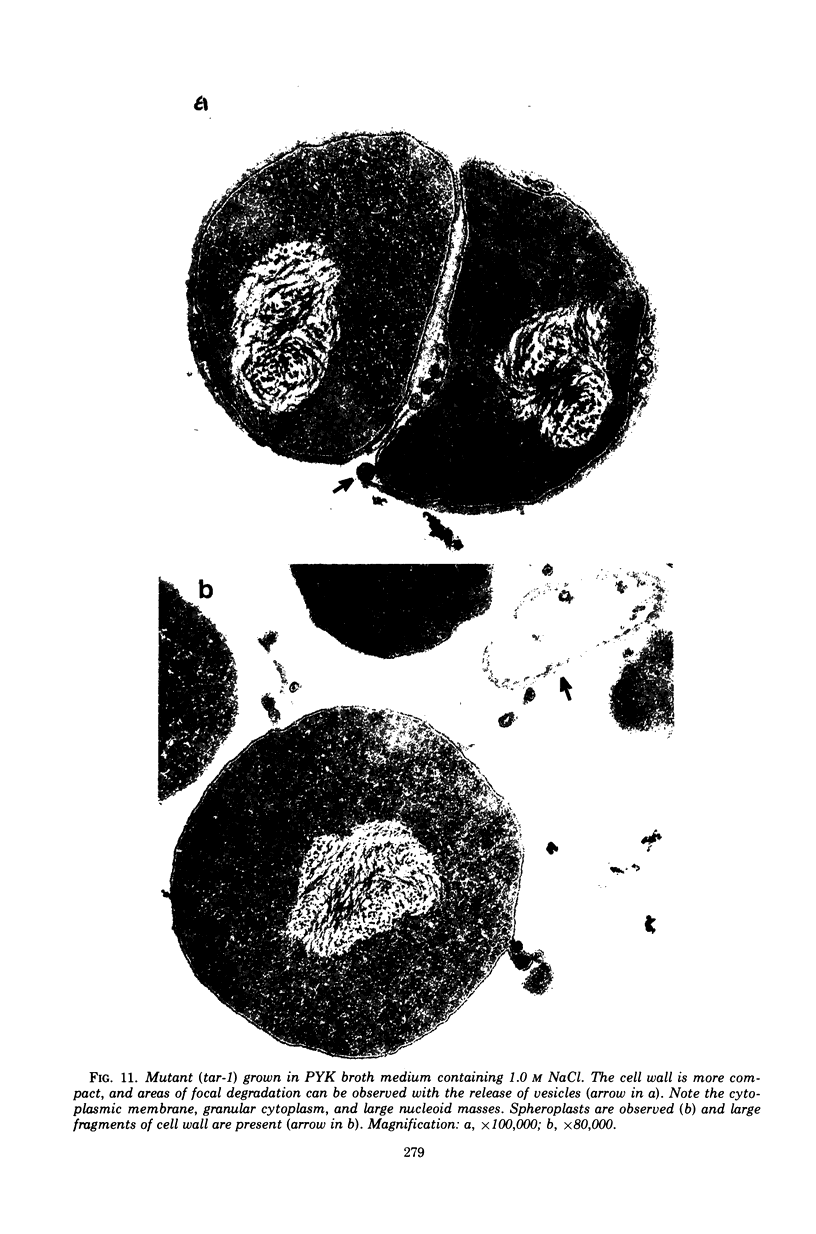
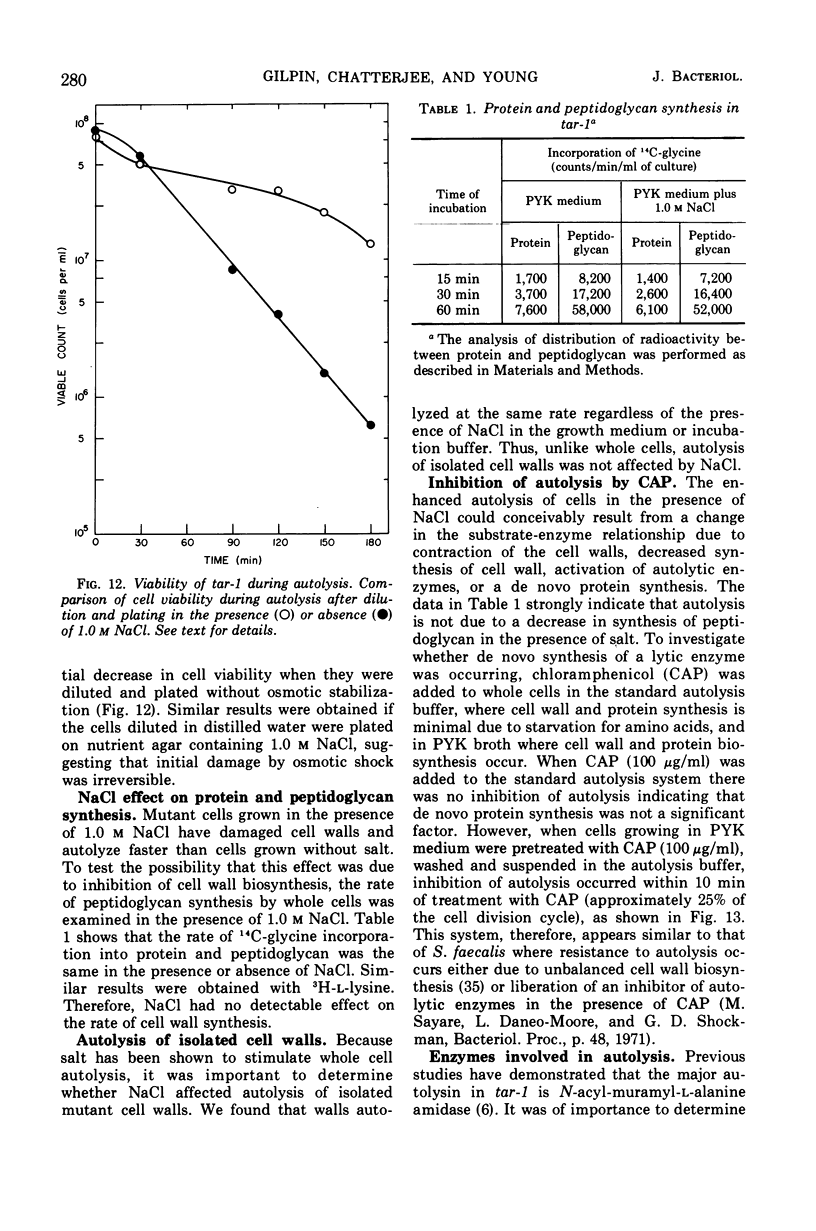
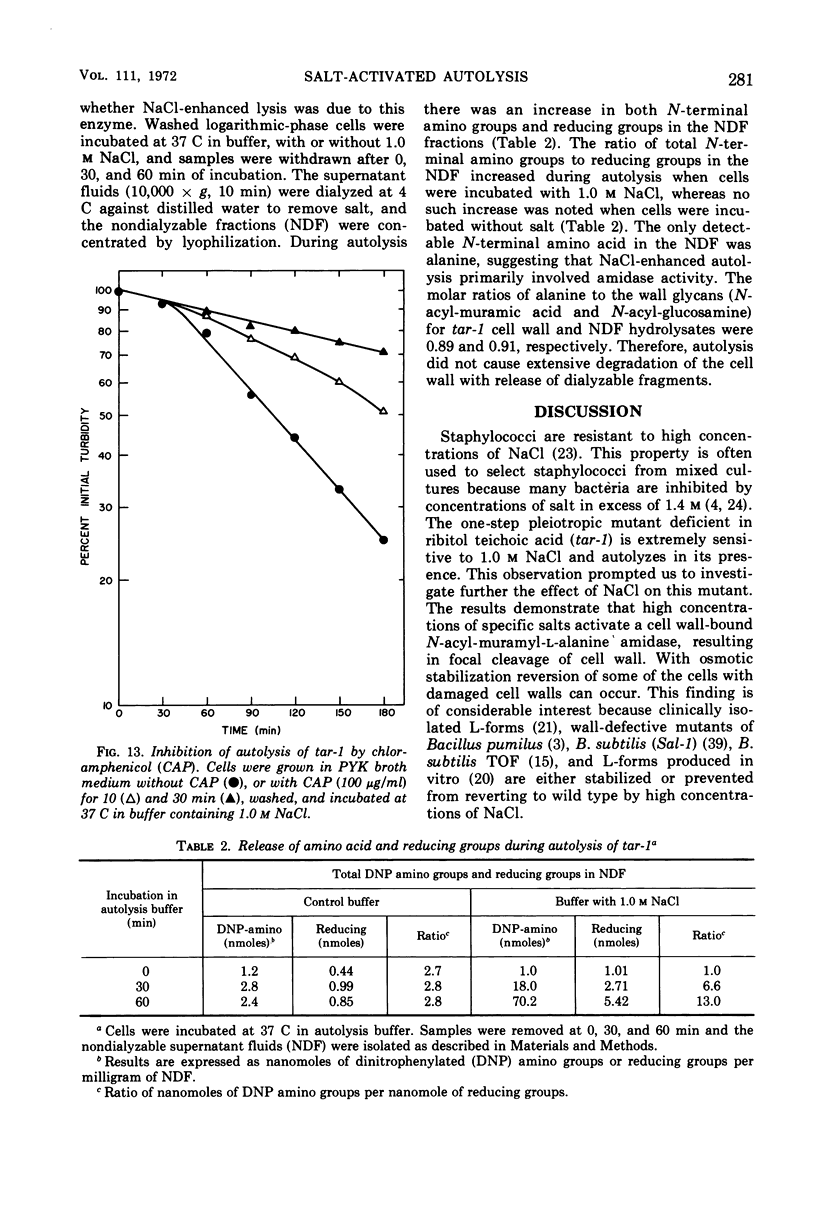
The effect of various salts on the autolysis of cell wall of a ribitol teichoic acid-deficient mutant of Staphylococcus aureus H (strain 52A5 carrying tar-1) was compared with the parent strain. In the presence of high concentrations of certain salts such as 1.0 m NaCl, the mutant undergoes autolysis with the release of osmotically sensitive spheroplasts. The parent strain is not affected by these conditions. The stimulation of lysis is related to an activation of N-acylmuramyl-l-alanine amidase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Burmeister H. R., Hesseltine C. W. Induction and propagation of a Bacillus subtilis L form in natural and synthetic media. J Bacteriol. 1968 May;95(5):1857–1861. doi: 10.1128/jb.95.5.1857-1861.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G. H. The Significance of Sodium Chloride in Studies of Staphylococci. J Bacteriol. 1945 Aug;50(2):201–203. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- De Voe I. W., Oginsky E. L. Antagonistic effect of monovalent cations in maintenance of cellular integrity of a marine bacterium. J Bacteriol. 1969 Jun;98(3):1355–1367. doi: 10.1128/jb.98.3.1355-1367.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Cell wall binding properties of the Bacillus subtilis autolysin(s). J Bacteriol. 1970 Aug;103(2):488–493. doi: 10.1128/jb.103.2.488-493.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Good C. M., Pattee P. A. Temperature-Sensitive Osmotically Fragile Mutants of Staphylococcus aureus. J Bacteriol. 1970 Dec;104(3):1401–1403. doi: 10.1128/jb.104.3.1401-1403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. H., Stow M., Hancock I. C., Baddiley J. Function of teichoic acids and effect of novobiocin on control of Mg2+ at the bacterial membrane. Nat New Biol. 1971 Jan 13;229(2):53–55. doi: 10.1038/newbio229053a0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Shaw D. R., Park J. T. Nature and origins of phosphorus compounds in isolated cell walls of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):239–244. doi: 10.1128/jb.107.1.239-244.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou L. T., Marquis R. E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970 Jan;101(1):92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. THE BACTERIAL CELL WALL. THE RESULT OF ADSORPTION, STRUCTURE OR SELECTIVE PERMEABILITY? J Gen Microbiol. 1963 Jul;32:19–24. doi: 10.1099/00221287-32-1-19. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]
- Young F. E., Haywood P., Pollock M. Isolation of L-forms of Bacillus subtilis which grow in liquid medium. J Bacteriol. 1970 Jun;102(3):867–870. doi: 10.1128/jb.102.3.867-870.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]