Abstract
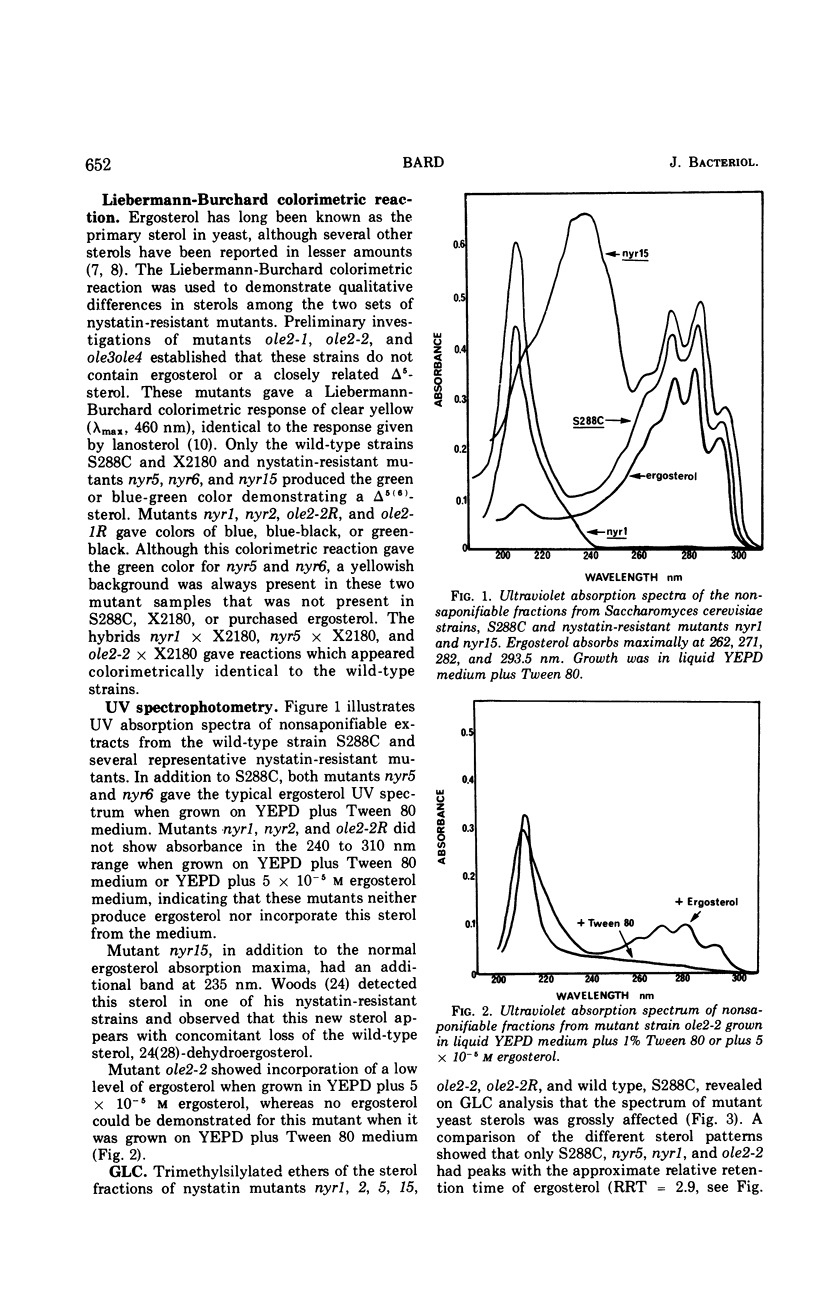
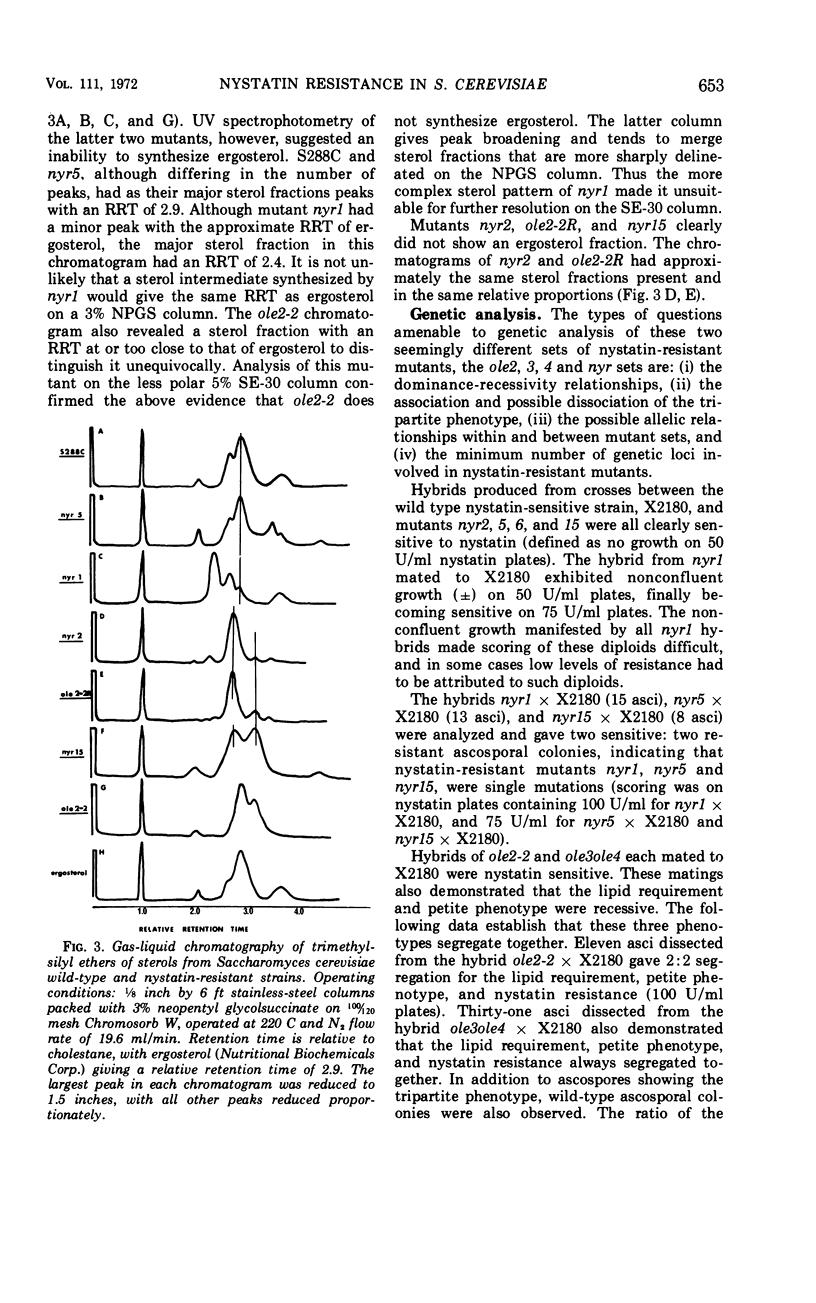
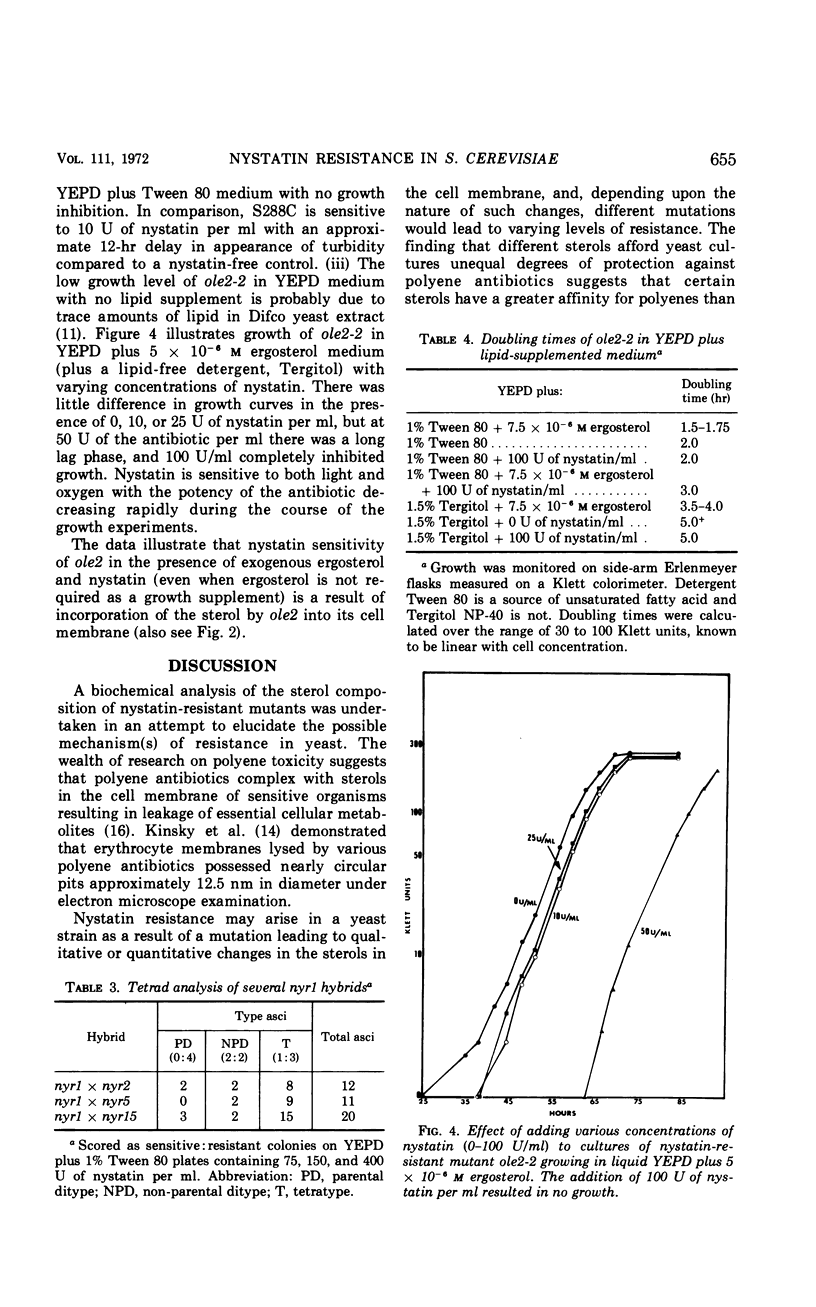
Two phenotypically distinct sets of nystatin-resistant mutants were investigated. One set is resistant, respiratory competent, and requires no lipid for growth. The other set is more resistant, respiratory deficient, and lipid requiring (unsaturated fatty acid or sterol). Both sets show altered sterol composition as demonstrated by the Liebermann-Burchard colorimetric reaction, ultraviolet spectrophotometry, and gas-liquid chromatography. Genetic analysis indicates that all nystatin-resistant mutants can be placed into one of six distinct genetic groups. The phenotype's nystatin resistance, lipid requirement, and respiratory deficiency are recessive. There was one case of allelism for mutants from different sets. Revertants of mutants which have the tripartite phenotype retain a residual level of nystatin resistance, but they are no longer lipid requiring or respiratory deficient. Growth studies in mutants which have the tripartite phenotype reveal that the addition of ergosterol to the growth medium results in decreased resistance to nystatin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Adams B. G., Parks L. W. Evidence for dual physiological formsof ergosterol in Saccharomyces cerevisiae. J Cell Physiol. 1967 Oct;70(2):161–168. doi: 10.1002/jcp.1040700205. [DOI] [PubMed] [Google Scholar]
- Ahmed K. A., Woods R. A. A genetic analysis of resistance to nystatin in Saccharomyces cerevisiae. Genet Res. 1967 Apr;9(2):179–193. doi: 10.1017/s0016672300010478. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Parvez M. A., Hunt P. F. Studies on the biosynthesis of the ergosterol side chain. Biochem J. 1969 Jul;113(4):727–732. doi: 10.1042/bj1130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulder C. J. Anaerobic growth, ergosterol content and sensitivity to a polyene antibiotic, of the yeast Schizosaccharomyces japonicus. Antonie Van Leeuwenhoek. 1971;37(3):353–358. doi: 10.1007/BF02218505. [DOI] [PubMed] [Google Scholar]
- GOTTLIEB D., CARTER H. E., SLONEKER J. H., AMMANN A. Protection of fungi against polyene antibiotics by sterols. Science. 1958 Aug 15;128(3320):361–361. doi: 10.1126/science.128.3320.361. [DOI] [PubMed] [Google Scholar]
- Goulston G., Goad L. J., Goodwin T. W. Sterol biosynthesis in fungi. Biochem J. 1967 Feb;102(2):15C–17C. doi: 10.1042/bj1020015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Resnick M. R., Haley A. B. Fatty acid desaturase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):415–420. doi: 10.1128/jb.98.2.415-420.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C., Luse S. A., van Deenen L. L. Interaction of polyene antibiotics with natural and artificial membrane systems. Fed Proc. 1966 Sep-Oct;25(5):1503–1510. [PubMed] [Google Scholar]
- MARINI F., ARNOW P., LAMPEN J. O. The effect of monovalent cations on the inhibition of yeast metabolism by nystatin. J Gen Microbiol. 1961 Jan;24:51–62. doi: 10.1099/00221287-24-1-51. [DOI] [PubMed] [Google Scholar]
- PARKS L. W., STARR P. R. A relationship between ergosterol and respiratory competency in yeast. J Cell Comp Physiol. 1963 Feb;61:61–65. doi: 10.1002/jcp.1030610107. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Mortimer R. K. Unsaturated fatty acid mutants of Saccharomyces cerevisiae. J Bacteriol. 1966 Sep;92(3):597–600. doi: 10.1128/jb.92.3.597-600.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARACHEK A., FOWLER G. L. Induction of heritable respiratory deficiency in Saccharomyces by pantothenate starvation. Nature. 1961 May 27;190:792–794. doi: 10.1038/190792a0. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Keith A. D., Resnick M. R. Double-bond requirement in a fatty acid desaturase mutant of Saccharomyces cerevisiae. J Bacteriol. 1970 Jan;101(1):160–165. doi: 10.1128/jb.101.1.160-165.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R. A. Nystatin-resistant mutants of yeast: alterations in sterol content. J Bacteriol. 1971 Oct;108(1):69–73. doi: 10.1128/jb.108.1.69-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


