Abstract
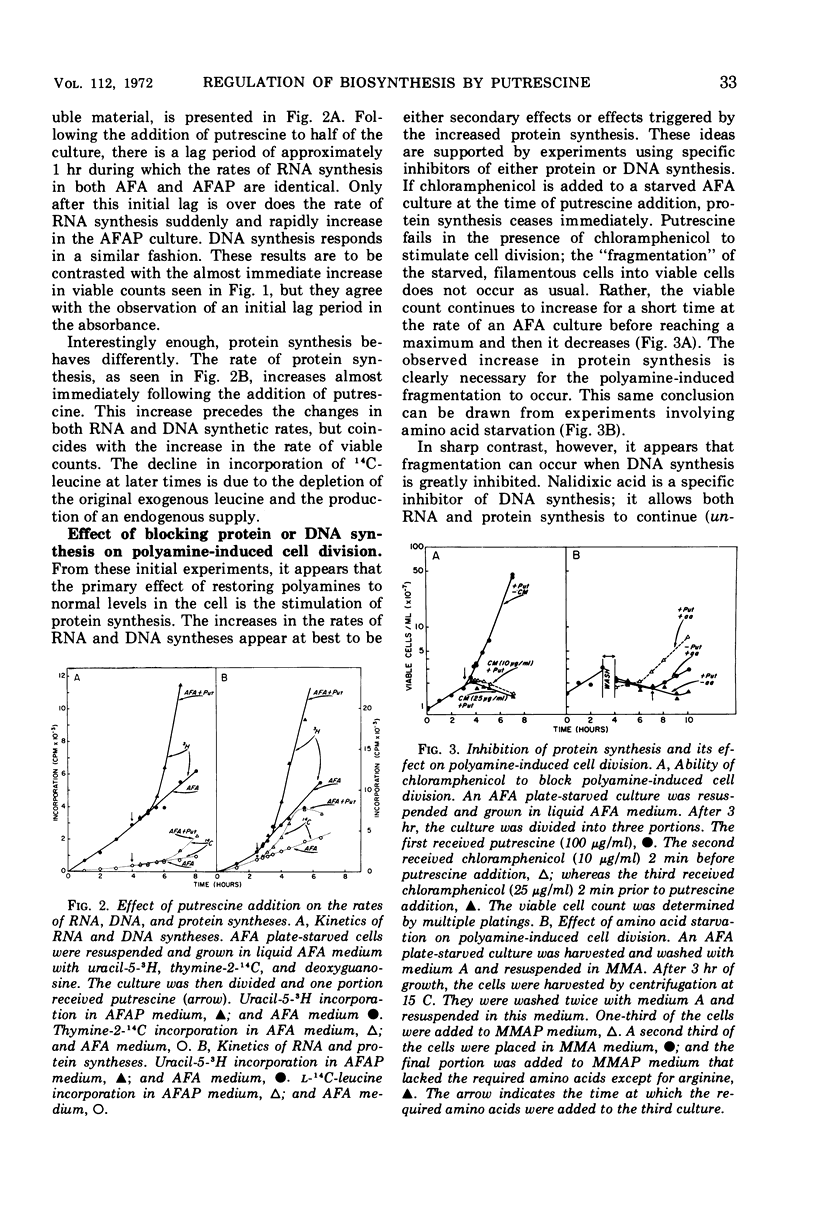
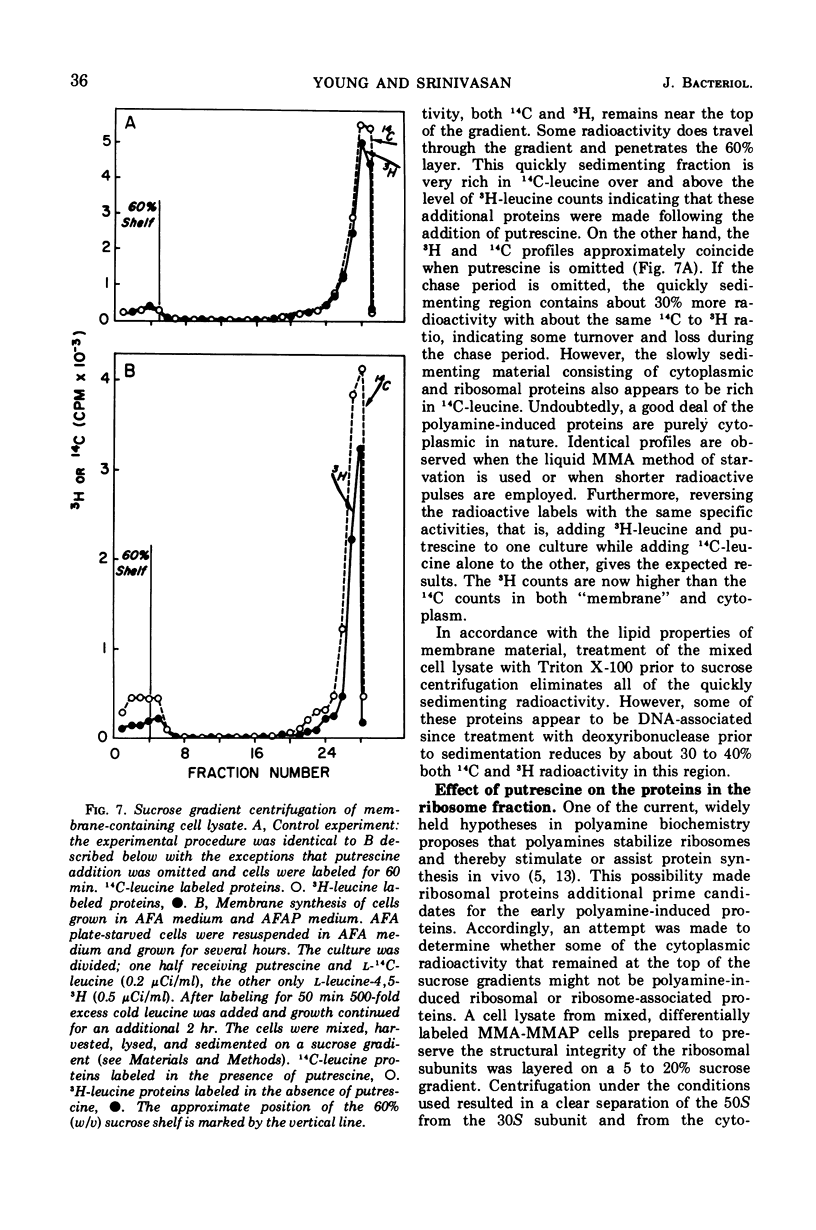
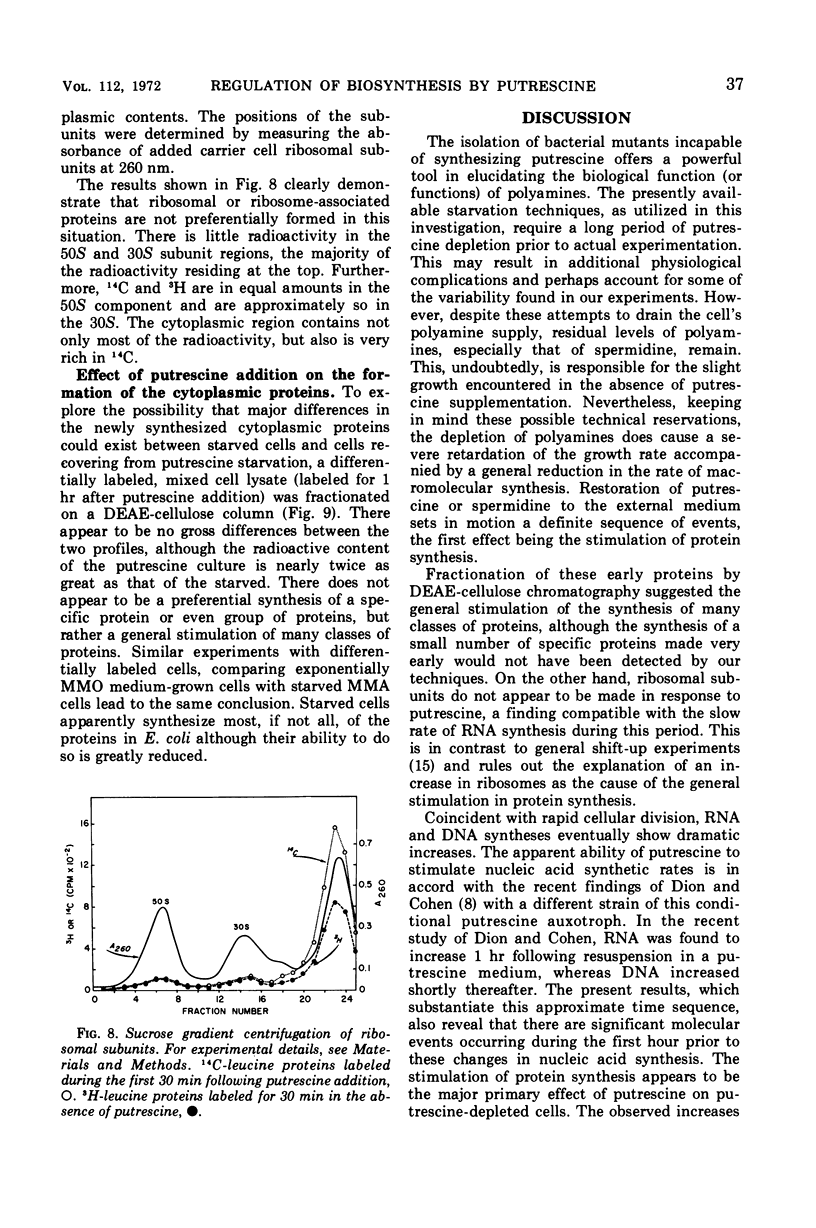
Addition of putrescine to a slowly growing, polyamine-starved Escherichia coli K-12 mutant conditionally incapable of synthesizing putrescine causes the immediate stimulation of protein synthesis. After a period ranging from 60 to 105 min, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) syntheses are also stimulated and rapid cellular division begins. Chloramphenicol blocks this rapid cellular division, although addition of the specific DNA synthesis inhibitor, nalidixic acid, has no effect on cell division. By sucrose gradient analysis and diethylaminoethyl-cellulose chromatography, the proteins initially made in response to putrescine appear to be composed of many classes, including membrane-bound proteins. However, the synthesis of ribosomal subunits is not altered during this period. A possible role for putrescine in either the stimulation of messenger RNA transcription or in translation is suggested.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Ben-Joseph M. Studies on ornithine decarboxylase activity in normal and T2-infected Escherichia coli. FEBS Lett. 1971 Jun 2;15(1):75–77. doi: 10.1016/0014-5793(71)80082-0. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- Changchien L. M., Aronson J. N. Spermidine requirement for Bacillus thuringiensis ribosomes in cell-free phenylalanine incorporation. J Bacteriol. 1970 Sep;103(3):734–740. doi: 10.1128/jb.103.3.734-740.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Hoffner N., Jansen M., Moore M., Raina A. POLYAMINES, RNA SYNTHESIS, AND STREPTOMYCIN LETHALITY IN A RELAXED MUTANT OF E. coli STRAIN 15 TAU. Proc Natl Acad Sci U S A. 1967 Mar;57(3):721–728. doi: 10.1073/pnas.57.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y. Association, profession, adaptation. Singapore Med J. 1971 Jun;12(3):121–126. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra W. G., Jr, Herbst E. J. Spermidine in Regenerating Liver: Relation to Rapid Synthesis of Ribonucleic Acid. Science. 1965 Jul 23;149(3682):428–429. doi: 10.1126/science.149.3682.428. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Turnock G. Stabilization of 70S ribosomes by spermidine. Nat New Biol. 1971 Jan 6;229(1):17–19. doi: 10.1038/newbio229017a0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G., Barbiroli B., Caldarera C. M. Polyamines and nucleic acid metabolism in chick embryo. Incorporation of labelled precursors into nucleic acids of subcellular fractions and polyribosomal patterns. Biochem J. 1968 May;107(5):609–613. doi: 10.1042/bj1070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J., Siimes M. Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim Biophys Acta. 1966 Jul 20;123(1):197–201. doi: 10.1016/0005-2787(66)90173-0. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Mizuno S., Maruo B. The binding of the parental DNA from bacteriophages to the cell membrane of Escherichia coli. Biochim Biophys Acta. 1971 Feb 25;232(1):14–20. doi: 10.1016/0005-2787(71)90486-2. [DOI] [PubMed] [Google Scholar]


