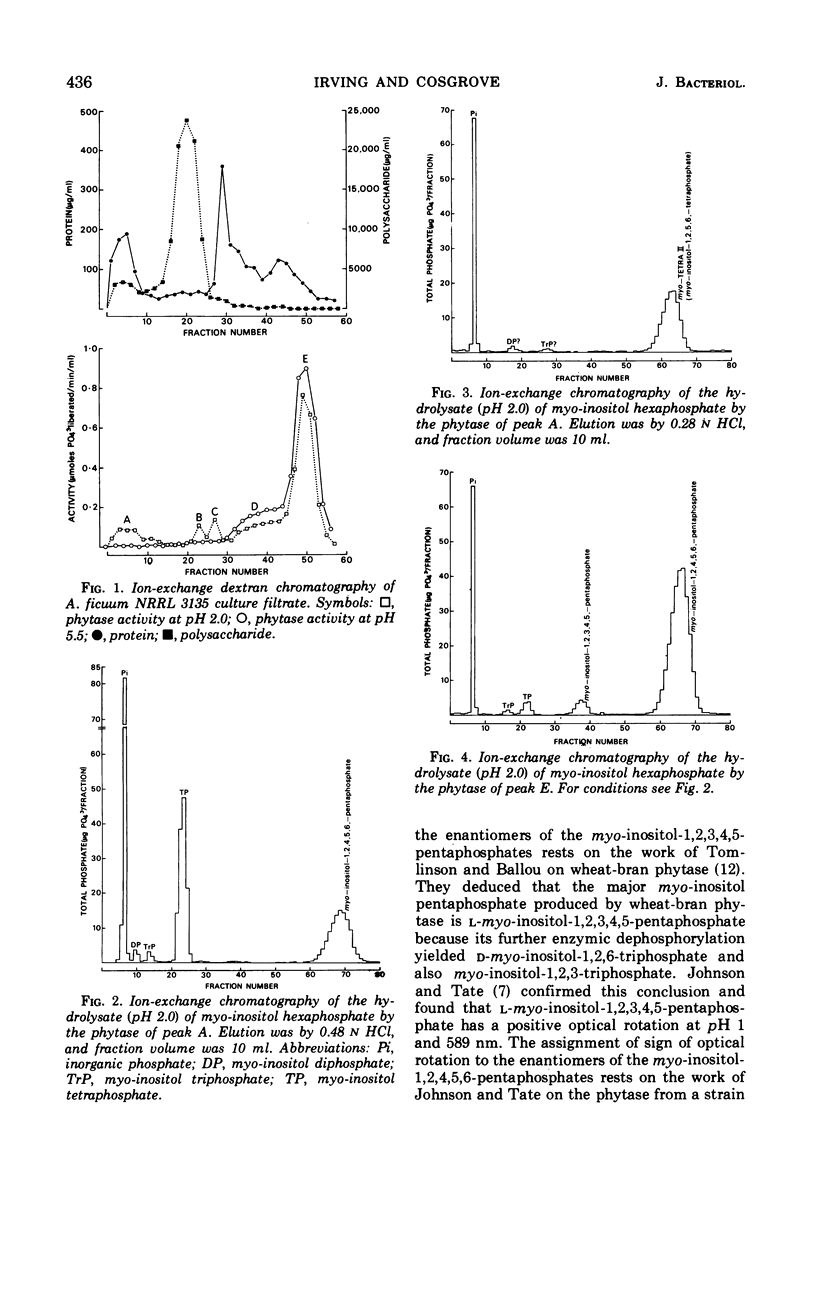
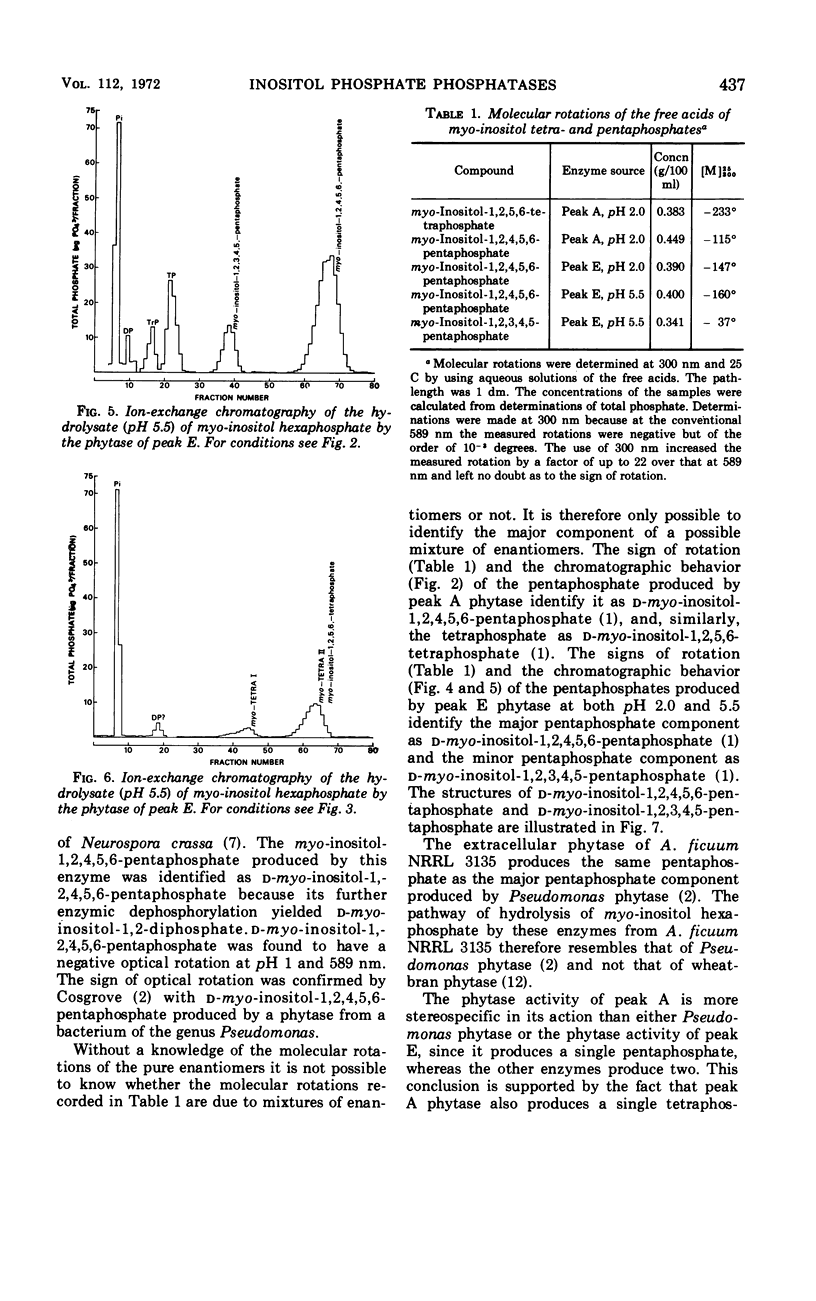
Abstract
The fungus Aspergillus ficuum NRRL 3135 is known to produce an extracellular nonspecific orthophosphoric monoester phosphohydrolase (EC 3.1.3.2) with a pH optimum of 2.0, as well as an extracellular myo-inositol hexaphosphate phosphohydrolase (EC 3.1.3.8; phytase) with pH optima of 2.0 and 5.5. Both these enzymes are also known to hydrolyze myo-inositol hexaphosphate. The pentaphosphates liberated in the first step of this hydrolysis have been isolated and identified by ion-exchange chromatography and optical rotation. The nonspecific orthophosphoric monoester phosphohydrolase produces a single pentaphosphate, d-myo-inositol-1,2,4,5,6-pentaphosphate, whereas the phytase, at both pH 2.0 and 5.5, produces a mixture of two pentaphosphates. The major component of this mixture is d-myo-inositol-1,2,4,5,6-pentaphosphate and the other is d-myo-inositol-1,2,3,4,5-pentaphosphate. Thus the pathways of dephosphorylation of myo-inositol hexaphosphate by these two enzymes differ from that of wheat-bran phytase which forms l-myo-inositol-1,2,3,4,5-pentaphosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cosgrove D. J. Inositol phosphate phosphatases of microbiological origin. Inositol phosphate intermediates in the dephosphorylation of the hexaphosphates of myo-inositol, scyllo-inositol, and D-chiro-inositol by a bacterial (Pseudomonas sp.) phytase. Aust J Biol Sci. 1970 Dec;23(6):1207–1220. doi: 10.1071/bi9701207. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J. Ion-exchange chromatography of inositol polyphosphates. Ann N Y Acad Sci. 1969 Oct 17;165(2):677–686. [PubMed] [Google Scholar]
- Irving G. C., Cosgrove D. J. Inositol phosphate phosphatases of microbiological origin. Observations on the nature of the active centre of a bacterial (Pseudomonas sp.) phytase. Aust J Biol Sci. 1971 Jun;24(3):559–564. doi: 10.1071/bi9710559. [DOI] [PubMed] [Google Scholar]
- Irving G. C., Cosgrove D. J. Inositol phosphate phosphatases of microbiological origin. Some properties of a partially purified bacterial (Pseudomonas sp.) phytase. Aust J Biol Sci. 1971 Jun;24(3):547–557. doi: 10.1071/bi9710547. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Tate M. E. The structure of myo-inositol pentaphosphates. Ann N Y Acad Sci. 1969 Oct 17;165(2):526–532. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Shieh T. R., Ware J. H. Survey of microorganism for the production of extracellular phytase. Appl Microbiol. 1968 Sep;16(9):1348–1351. doi: 10.1128/am.16.9.1348-1351.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh T. R., Wodzinski R. J., Ware J. H. Regulation of the formation of acid phosphatases by inorganic phosphate in Aspergillus ficuum. J Bacteriol. 1969 Dec;100(3):1161–1165. doi: 10.1128/jb.100.3.1161-1165.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON R. V., BALLOU C. E. Myoinositol polyphosphate intermediates in the dephosphorylation of phytic acid by phytase. Biochemistry. 1962 Jan;1:166–171. doi: 10.1021/bi00907a025. [DOI] [PubMed] [Google Scholar]


