Abstract
Some kinetic properties of the adenine phosphoribosyltransferases from Escherichia coli and Mycoplasma mycoides have been studied. For the E. coli enzyme, Michaelis constants for adenine and 5-phosphoribosyl-1-pyrophosphate (PRPP) are 1.3 and 10 μm, respectively. Adenosine monophosphate, the most effective nucleotide inhibitor, inhibits competitively with respect to PRPP, the inhibition constant being 26 μm. The M. mycoides enzyme has more complex kinetics. The response to increasing PRPP concentration is sigmoidal, the degree of sigmoidality depending on both the concentration of adenine and the pH. At low PRPP levels, high concentrations of adenine are inhibitory. Guanosine monophosphate is the most effective inhibitor, being inhibitory at all pH values, but other nucleotides have been found to activate at pH 7 and inhibit at pH 8. The elution profile of the M. mycoides enzyme from Sephadex suggests an association of enzyme subunits in the presence of PRPP. This is consistent with the observed kinetics if the associated form has increased stability and activity.
Full text
PDF

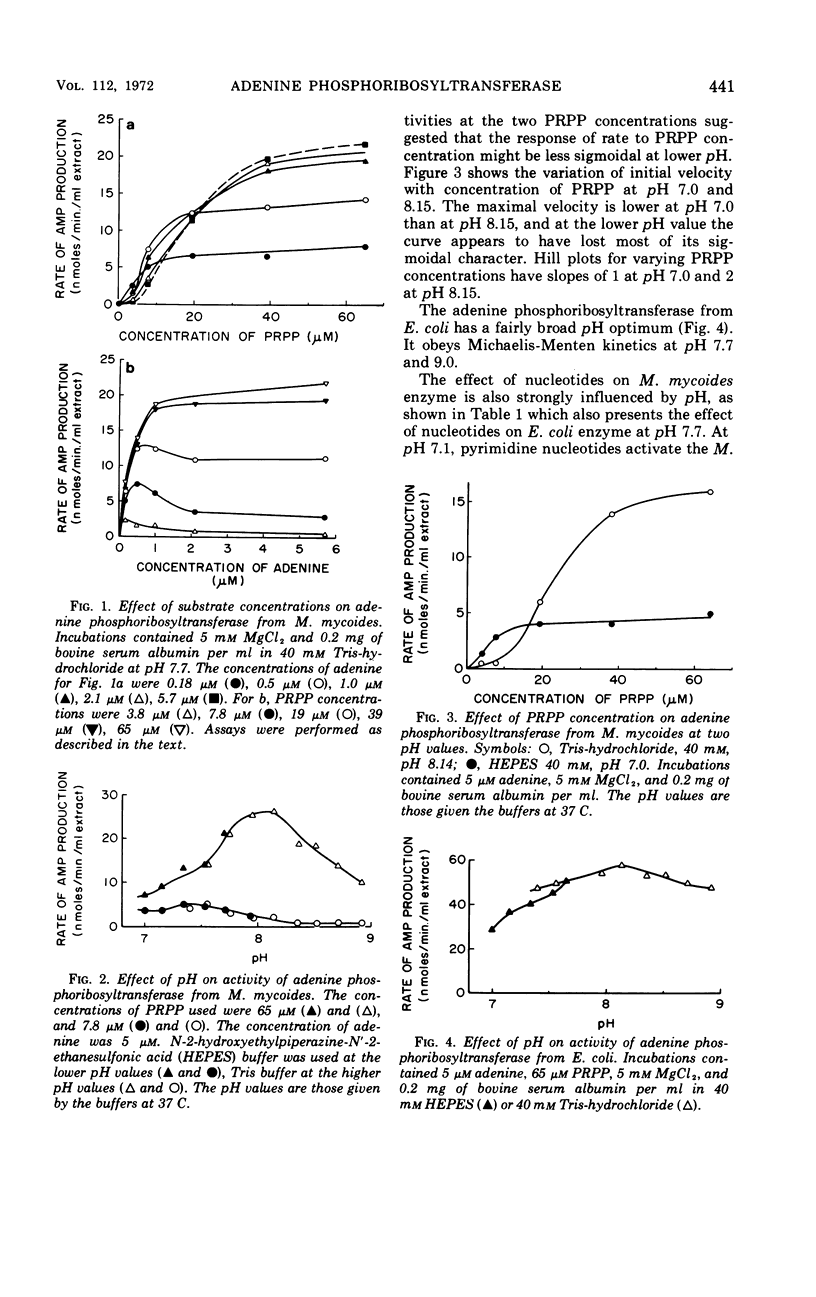
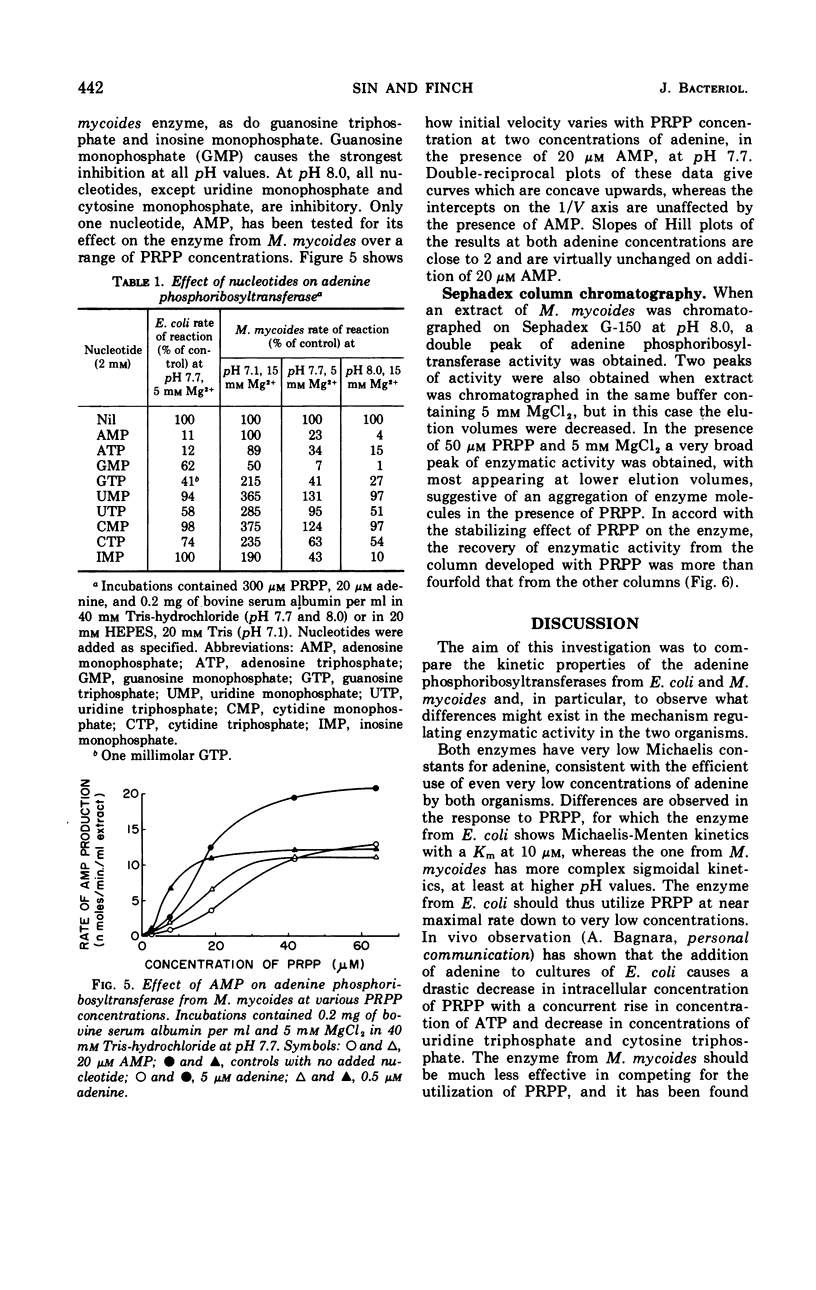
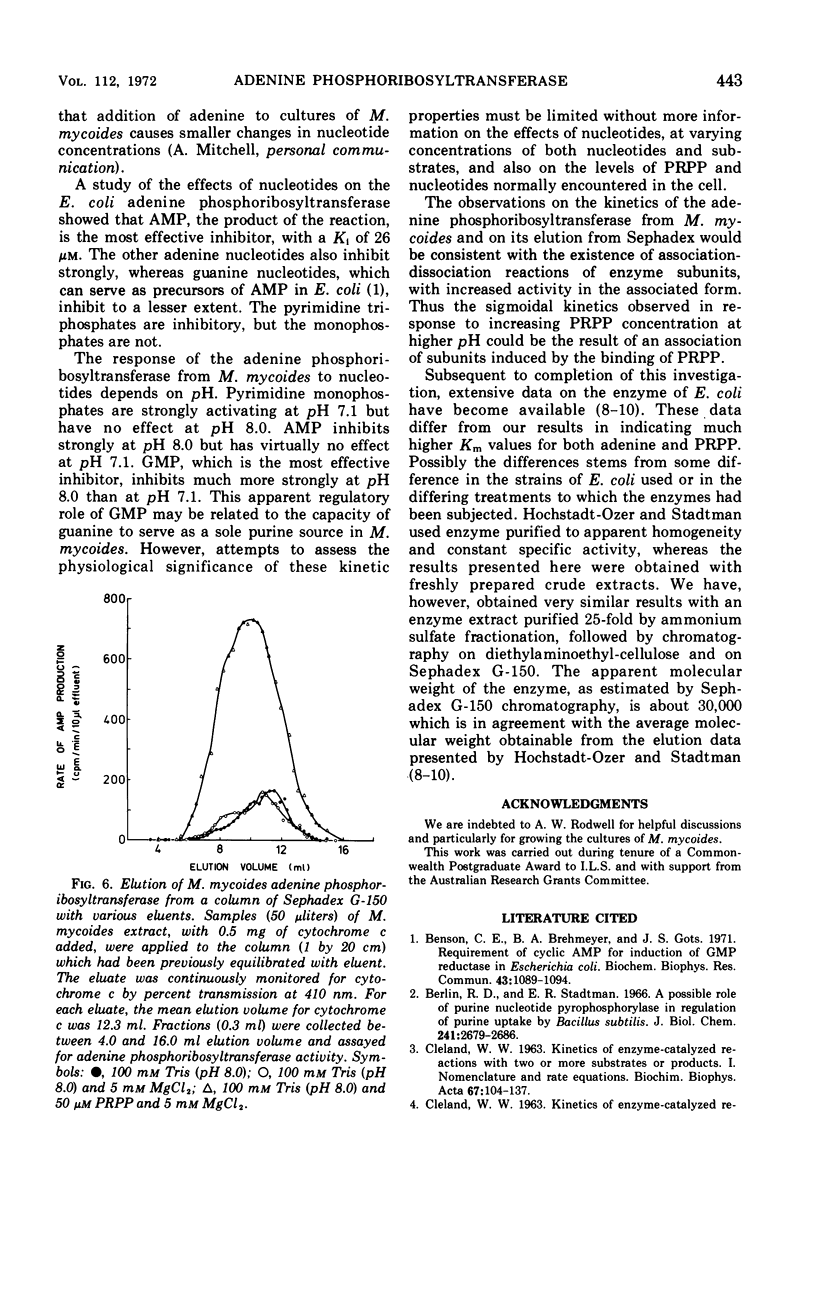

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson C. E., Brehmeyer B. A., Gots J. S. Requirement of cyclic AMP for induction of GMP reductase in Escherichia coli. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1089–1094. doi: 10.1016/0006-291x(71)90573-0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Stadtman E. R. A possible role of purine nucleotide pyrophosphorylases in the regulation of purine uptake by Bacillus subtilis. J Biol Chem. 1966 Jun 10;241(11):2679–2686. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. I. Purification of adenine phosphoribosyltransferase from Escherichia coli K12 and control of activity by nucleotides. J Biol Chem. 1971 Sep 10;246(17):5294–5303. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. III. The involvement of purine phosphoribosyltransferases in the uptake of adenine and other nucleic acid precursors by intact resting cells. J Biol Chem. 1971 Sep 10;246(17):5312–5320. [PubMed] [Google Scholar]
- Hori M., Henderson J. F. Purification and properties of adenylate pyrophosphorylase from Ehrlich ascites tumor cells. J Biol Chem. 1966 Mar 25;241(6):1406–1411. [PubMed] [Google Scholar]
- KALLE G. P., GOTS J. S. GENETIC ALTERATION OF ADENYLIC PYROPHOSPHORYLASE IN SALMONELLA. Science. 1963 Nov 8;142(3593):680–681. doi: 10.1126/science.142.3593.680. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., LIEBERMAN I., SIMMS E. S. Enzymatic synthesis of purine nucleotides. J Biol Chem. 1955 Jul;215(1):417–427. [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. Adenine phosphoribosyltransferase from monkey liver. Specificity and properties. J Biol Chem. 1969 Sep 10;244(17):4779–4784. [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. 3. On the regulation of the deoxyadenosine triphosphate and deoxycytidine triphosphate pools of Escherichia coli. Biochim Biophys Acta. 1966 Oct 24;129(1):104–115. doi: 10.1016/0005-2787(66)90012-8. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Rodwell A. W. The nutrition and metabolism of mycoplasma: Progress and problems. Ann N Y Acad Sci. 1967 Jul 28;143(1):88–109. doi: 10.1111/j.1749-6632.1967.tb27649.x. [DOI] [PubMed] [Google Scholar]
- Rodwell A. W. The supply of cholesterol and fatty acids for the growth of mycoplasmas. J Gen Microbiol. 1969 Sep;58(1):29–37. doi: 10.1099/00221287-58-1-29. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WAY J. L., PARKS R. E., Jr Enzymatic synthesis of 5'-phosphate nucleotides of purine analogues. J Biol Chem. 1958 Mar;231(1):467–480. [PubMed] [Google Scholar]


