Abstract
The phosphotransacetylase from Clostridium acidiurici has two properties not observed for this enzyme in other bacteria: (i) it requires a divalent metal for activity, and (ii) it is not subject to uncoupling by arsenate. The enzyme has been obtained in highly purified form, with a specific activity 500-fold higher than crude extracts. Ferrous or manganous ions are required for maximal activity, with Mn2+ being 50 to 75% as effective as Fe2+. The acetyl group can be transferred from acetyl phosphate to coenzyme A in 20 mm arsenate without a net decrease in high-energy acyl linkages. Likewise, H32PO42− will exchange with acetyl-PO42− in the presence of arsenate without loss of acetyl phosphate. This suggests that the active site on the enzyme is capable of discriminating between phosphate and arsenate while permitting the reversible transfer of acyl groups between CoA and phosphate.
Full text
PDF


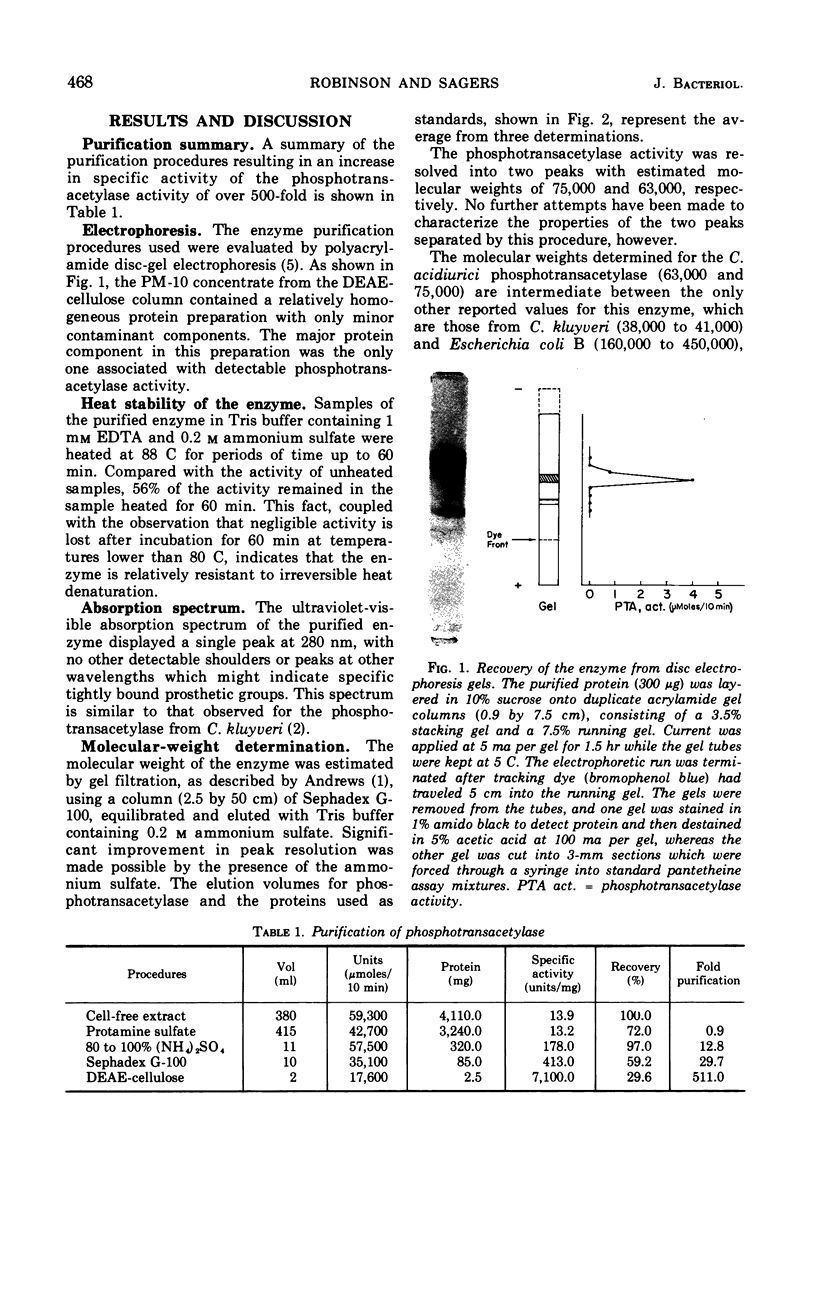
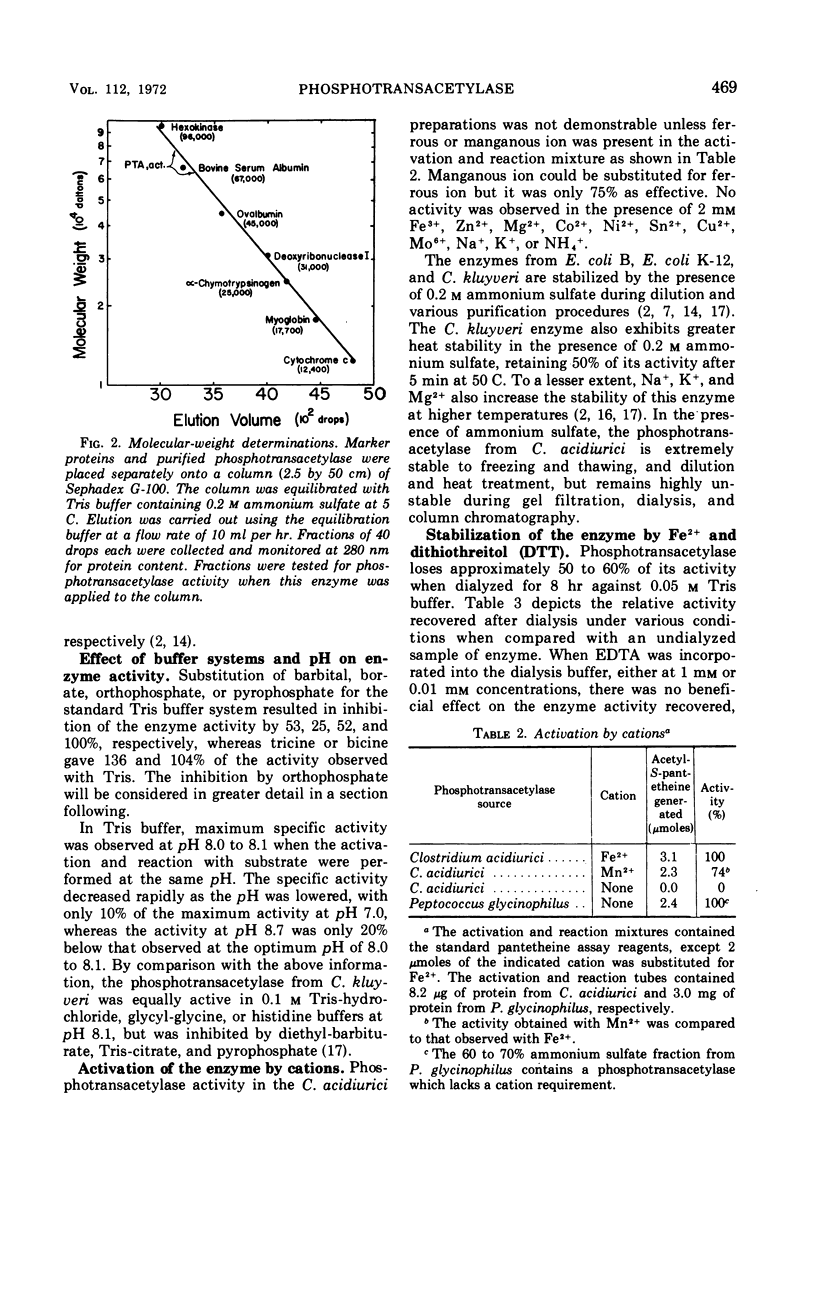
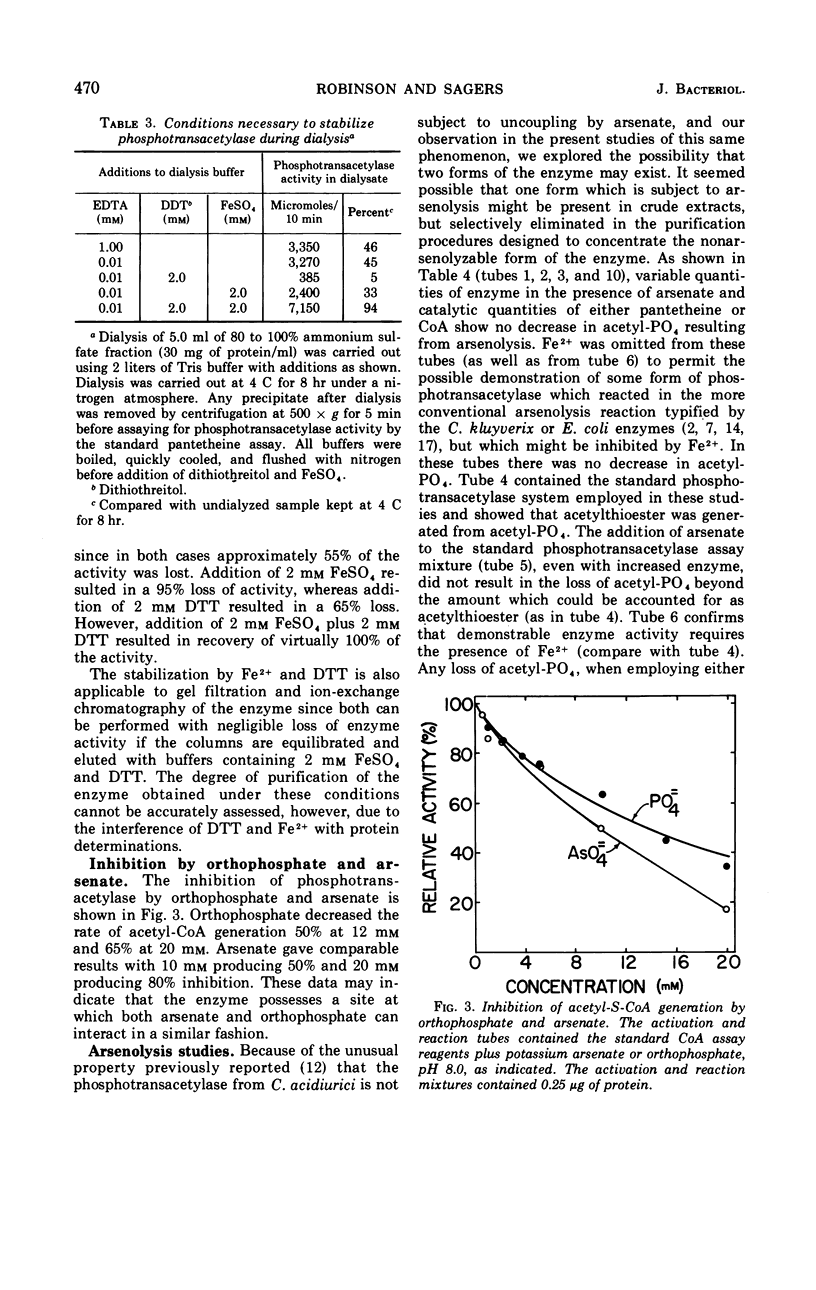

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dann O., Roselieb H., Sucker H. Acetokinase, Phosphotransacetylasen und Cholinacetylase der Honigbiene. Justus Liebigs Ann Chem. 1967;710:161–170. doi: 10.1002/jlac.19677100117. [DOI] [PubMed] [Google Scholar]
- Dann O., Sucker H. Phosphotransacetylase und biogene Hemmstoffe der Acetat-Aktivierung bzw. der Acetylphosphatase im Blutegel (Hirudo medicinalis) Justus Liebigs Ann Chem. 1967;705:238–255. doi: 10.1002/jlac.19677050126. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Purification of phosphotransacetylase from Escherichia coli, K-12. Biochim Biophys Acta. 1958 May;28(2):436–437. doi: 10.1016/0006-3002(58)90493-1. [DOI] [PubMed] [Google Scholar]
- KLEIN S. M., SAGERS R. D. Intermediary metabolism of Diplococcus glycinophilus. II. Enzymes of the acetategenerating system. J Bacteriol. 1962 Jan;83:121–126. doi: 10.1128/jb.83.1.121-126.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- SAGERS R. D., BENZIMAN M., KLEIN S. M. FAILURE OF ARSENATE TO UNCOUPLE THE PHOSPHOTRANSACETYLASE SYSTEM IN CLOSTRIDIUM ACIDIURICI. J Bacteriol. 1963 Nov;86:978–984. doi: 10.1128/jb.86.5.978-984.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLY W. S., STADTMAN E. R. FORMATE METABOLISM. II. ENZYMATIC SYNTHESIS OF FORMYL PHOSPHATE AND FORMYL COENZYME A IN CLOSTRIDIUM CYLINDROSPORUM. J Biol Chem. 1963 Aug;238:2639–2647. [PubMed] [Google Scholar]
- STADTMAN E. R., LIPMANN F. Acetyl phosphate synthesis by reaction of isopropenyl acetate and phosphoric acid. J Biol Chem. 1950 Aug;185(2):549–551. [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- SUCKER H. ACETOKINASE UND PHOSPHOTRANSACETYLASE DER STUBENFLIEGE. Justus Liebigs Ann Chem. 1965 Mar;683:225–238. doi: 10.1002/jlac.19656830127. [DOI] [PubMed] [Google Scholar]
- SUCKER H. ACETYLCHOLIN. X. ACETOKINASE UND PHSPHOTRANSACETYLASE DE HEFE. Justus Liebigs Ann Chem. 1965 Mar;683:239–254. doi: 10.1002/jlac.19656830128. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Suzuki T., Kameda K. Y., Abiko Y. Phosphotransacetylase of Escherichia coli B, purification and properties. Biochim Biophys Acta. 1969;191(3):550–558. doi: 10.1016/0005-2744(69)90348-9. [DOI] [PubMed] [Google Scholar]
- TWAROG R., WOLFE R. S. ROLE OF BUTYRYL PHOSPHATE IN THE ENERGY METABOLISM OF CLOSTRIDIUM TETANOMORPHUM. J Bacteriol. 1963 Jul;86:112–117. doi: 10.1128/jb.86.1.112-117.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Purification and role of phosphotransbutyrylase. J Biol Chem. 1960 Jul;235:1948–1952. [PubMed] [Google Scholar]



