Abstract
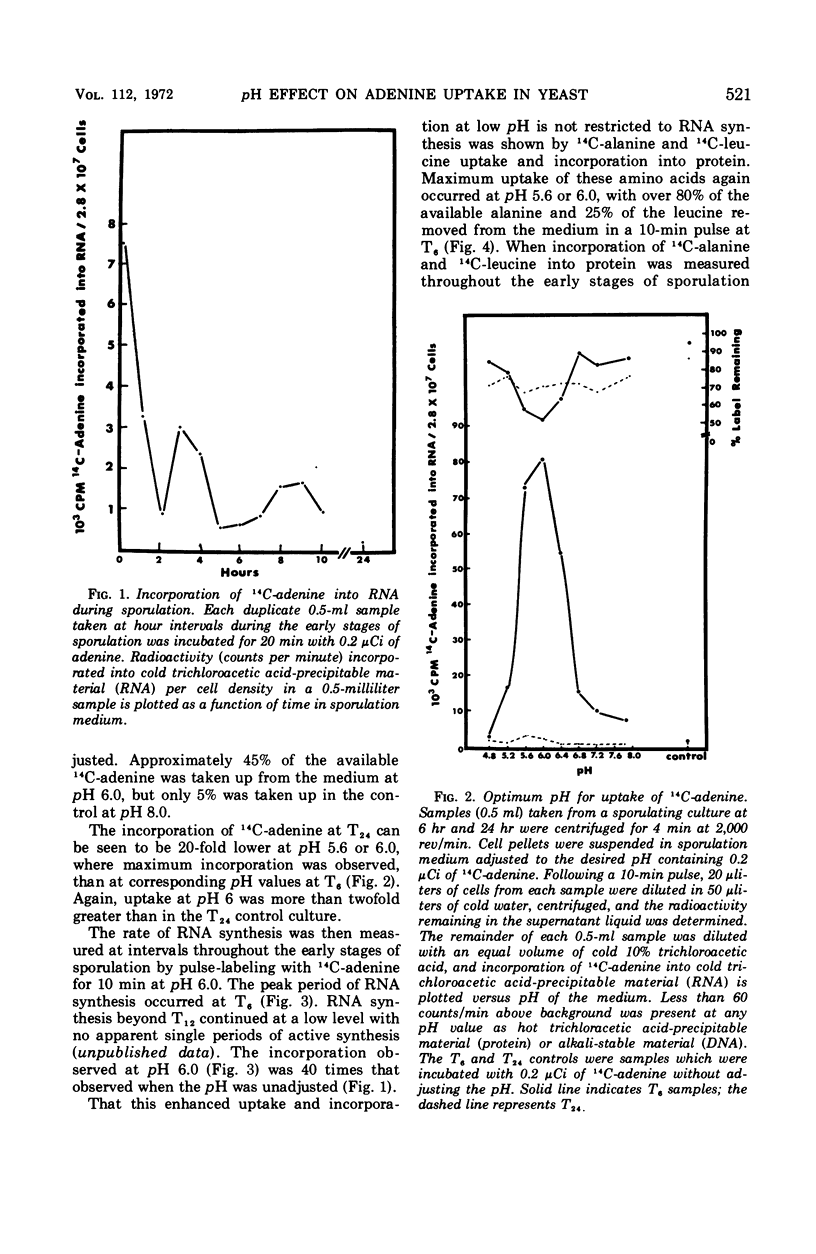
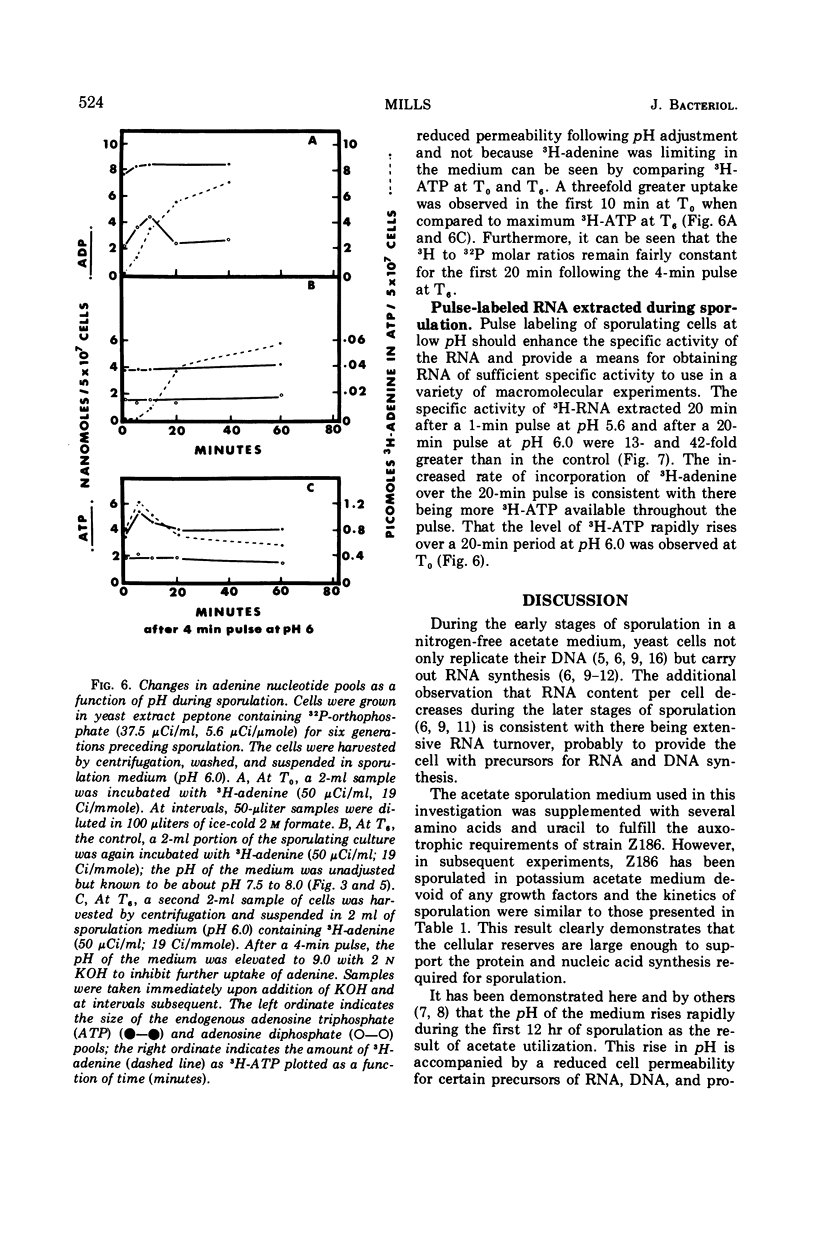
During the early stages of sporulation in Saccharomyces cerevisiae, the pH of the acetate sporulation medium rises to values of 8.0 or higher. Associated with this rise in pH is a reduced cell permeability to certain precursors of ribonucleic acid (RNA), deoxyribonucleic acid or protein. Uptake of adenine, alanine, and leucine was optimal at pH 5.6 to 6.0, but sporulation was inhibited when the sporulation medium was buffered below pH 7.0. Cellular impermeability can be largely overcome by adjusting the acetate sporulation medium to pH 6.0 for optimal uptake of 14 C-adenine during short pulses without any apparent effect on sporulation. Sporulating cells pulse-labeled 20 min at pH 6.0 incorporated 40 times more 14C-adenine into RNA than sporulating cells pulse-labeled at pH 8.0. This increased incorporation can be attributed to a 100-fold increase in labeled adenosine triphosphate in cells pulse-labeled at pH 6.0 where maximum uptake occurs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Cashel M., Gallant J. Control of RNA synthesis in Escherichia coli. I. Amino acid dependence of the synthesis of the substrates of RNA polymerase. J Mol Biol. 1968 Jul 14;34(2):317–330. doi: 10.1016/0022-2836(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Croes A. F. Duplication of DNA during meiosis in baker's yeast. Exp Cell Res. 1966 Feb;41(2):452–454. doi: 10.1016/s0014-4827(66)80151-9. [DOI] [PubMed] [Google Scholar]
- DUGGAN P. F. THE UPTAKE OF POTASSIUM BY BAKER'S YEAST FROM POTASSIUM ACETATE. Biochim Biophys Acta. 1964 Jul 29;88:223–224. doi: 10.1016/0926-6577(64)90174-3. [DOI] [PubMed] [Google Scholar]
- EATON N. R. New press for disruption of microorganisms. J Bacteriol. 1962 Jun;83:1359–1360. doi: 10.1128/jb.83.6.1359-1360.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Appearance of a new species of ribonucleic acid during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):826–830. doi: 10.1128/jb.105.3.826-830.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Isolation and properties of a new species of ribonucleic acid synthesized in sporulating cells of Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):831–836. doi: 10.1128/jb.105.3.831-836.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- Tisdale J. H., DeBusk A. G. Developmental regulation of amino acid transport in Neurospora crassa. J Bacteriol. 1970 Nov;104(2):689–697. doi: 10.1128/jb.104.2.689-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


